Synthesis of a Novel Porphyrin-Based Metal–Organic Framework (Co-Por MOF) †
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Synthesis of TCPP
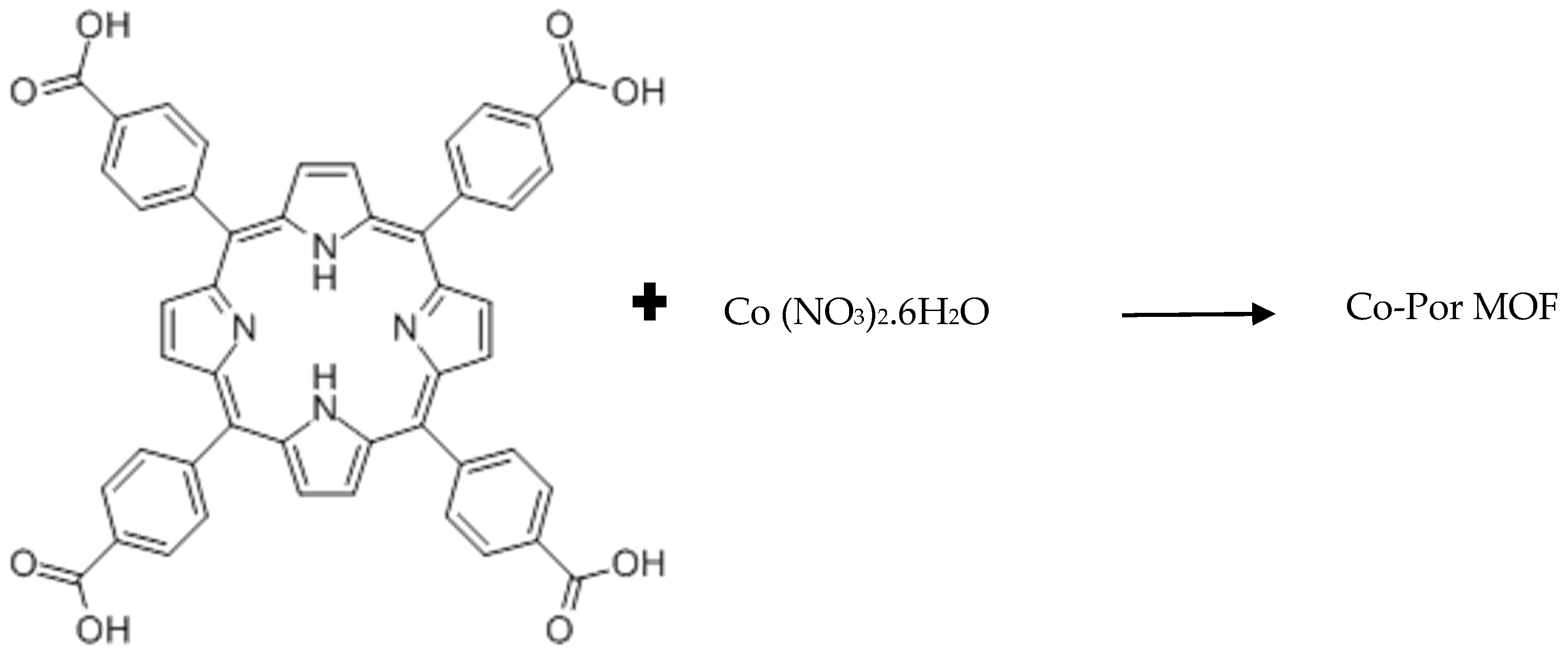
2.3. Synthesis of Co-Por MOF
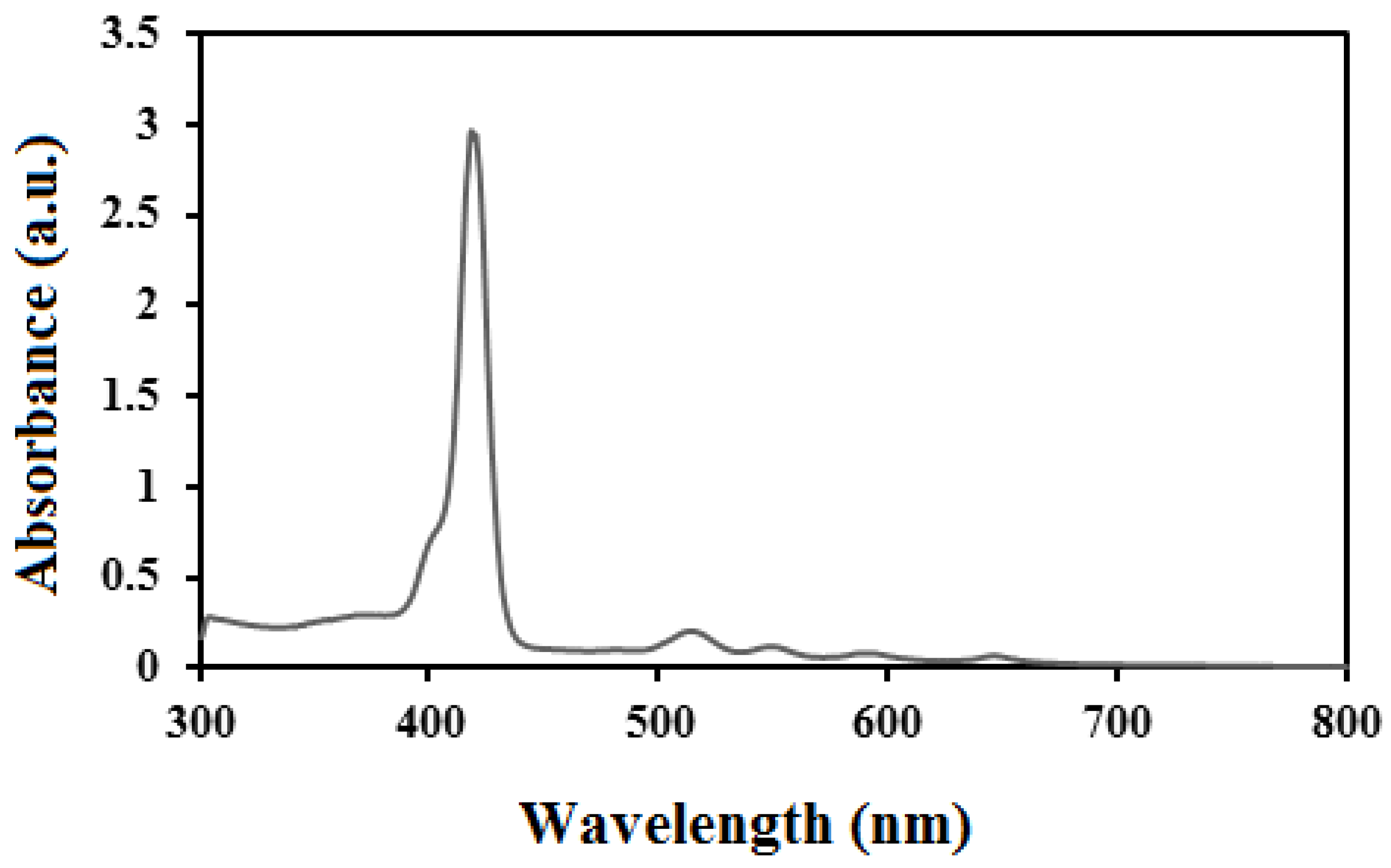
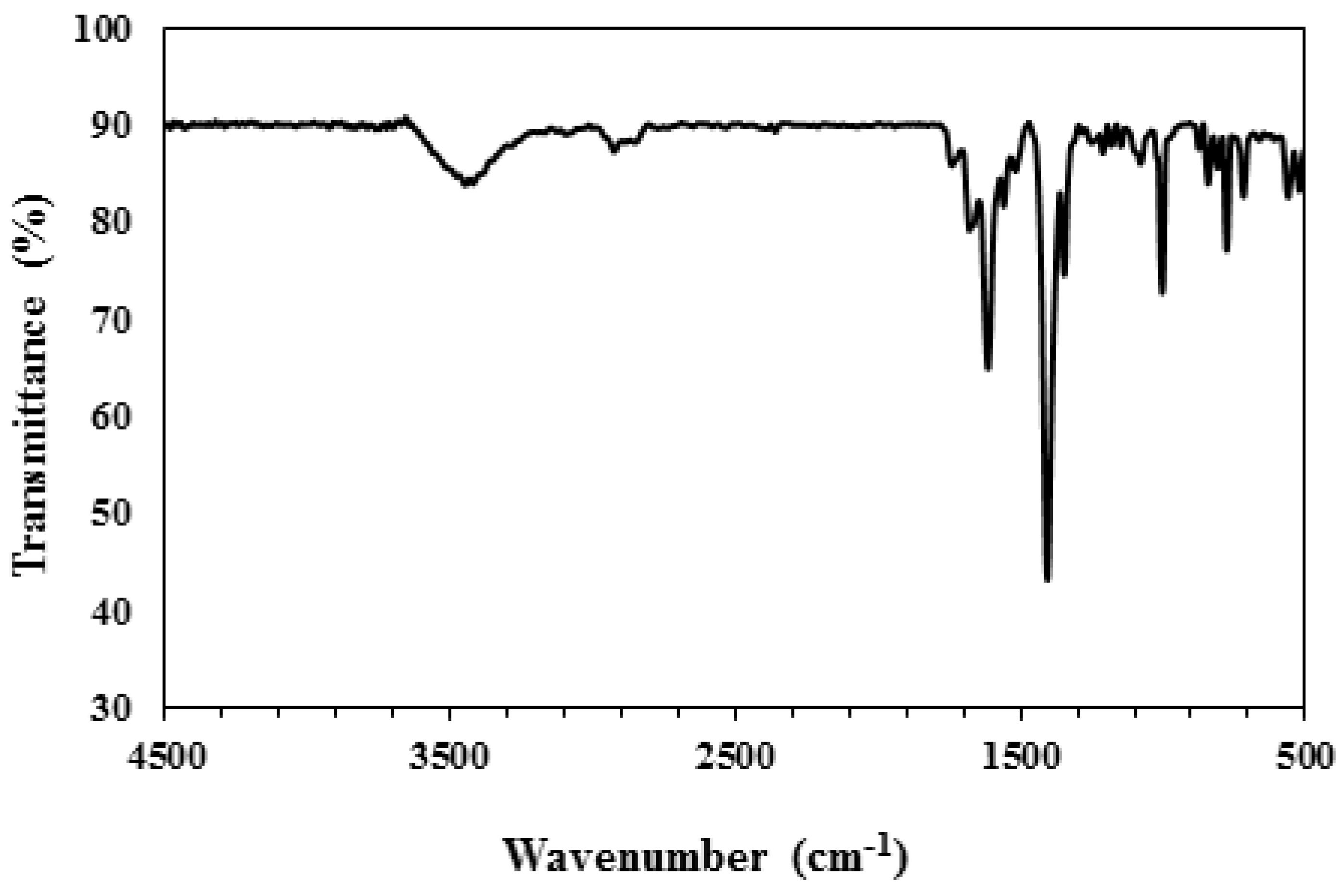
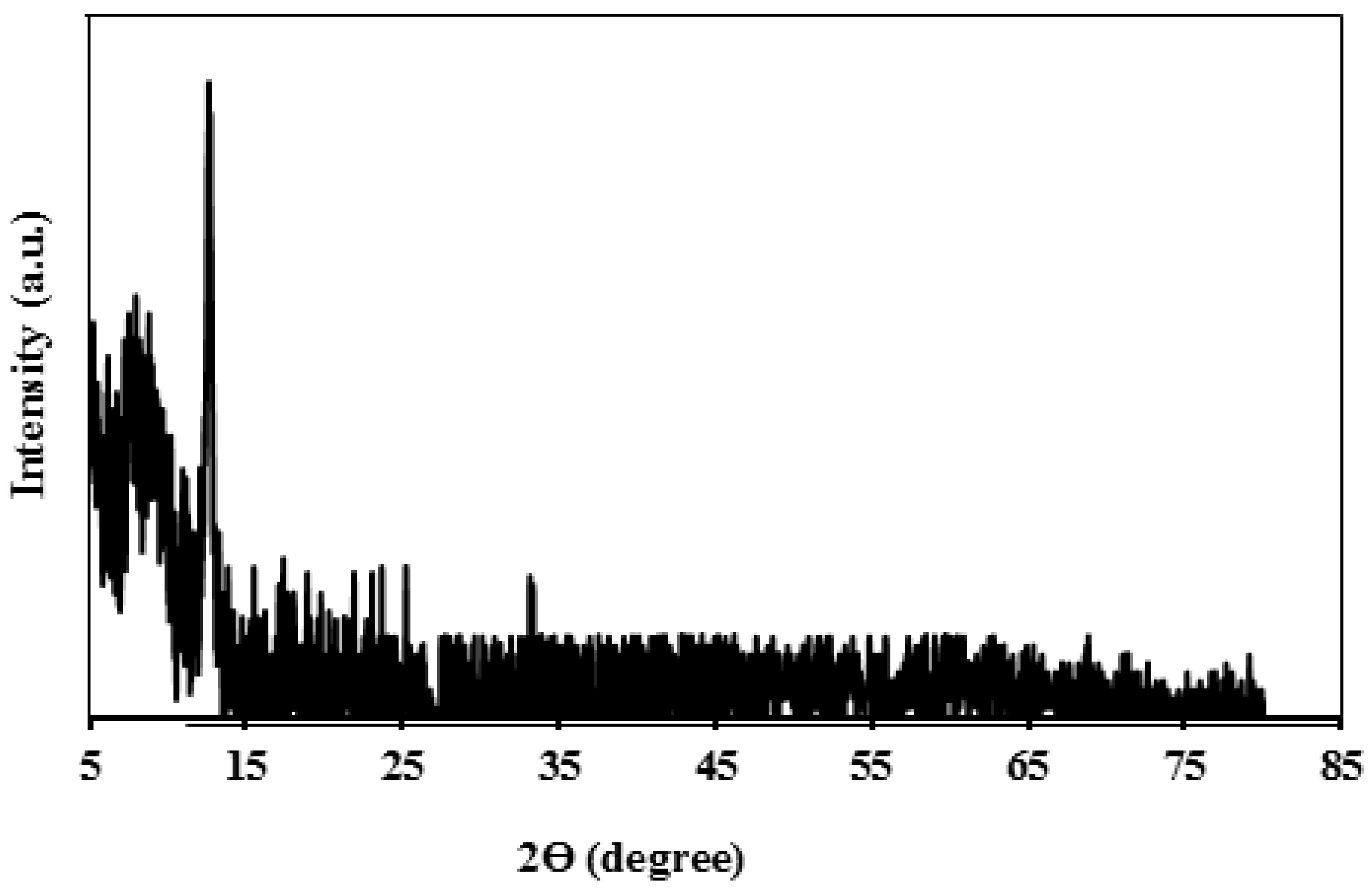
3. Characterization
4. Result and Discussion
5. Conclusions
References
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280. [Google Scholar] [CrossRef]
- Deria, P.; Chung, Y.G.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Water stabilization of Zr 6-based metal–organic frameworks via solvent-assisted ligand incorporation. Chem. Sci. 2015, 6, 5172–5176. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Millward, R.A.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@ cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-L.; Wang, K.; Wang, B.; Su, J.; Zou, X.; Xie, Y.; Li, J.; Zhou, H. A base-resistant metalloporphyrin metal–organic framework for C–H bond halogenation. J. Am. Chem. Soc. 2016, 139, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Qin, C.; Wang, X.L.; Su, Z.M. Metal-organic frameworks as potential drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X. Multifunctional metal–organic frameworks for photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 2762–2788. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.P. Synthesis and Characterization of Porphyrin Containing Metal-Organic Frameworks; Diss. UC: San Diego, CA, USA, 2013. [Google Scholar]
- Feng, D.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.; Zhou, H.C. Zirconium-metalloporphyrin PCN-222: Mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef]
- Heidari-Golafzani, R.M.M.; Rahimi, R. Synthesis of TCPP/ZnFe2O4@ ZnO nanohollow sphere composite for degradation of methylene blue and 4-nitrophenol under visible light. Mater. Chem. Phys. 2016, 179, 35–41. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayebi, L.; Rahimi, R.; Rabbani, M. Synthesis of a Novel Porphyrin-Based Metal–Organic Framework (Co-Por MOF). Proceedings 2019, 41, 83. https://doi.org/10.3390/ecsoc-23-06483
Tayebi L, Rahimi R, Rabbani M. Synthesis of a Novel Porphyrin-Based Metal–Organic Framework (Co-Por MOF). Proceedings. 2019; 41(1):83. https://doi.org/10.3390/ecsoc-23-06483
Chicago/Turabian StyleTayebi, Leyla, Rahmatollah Rahimi, and Mahboubeh Rabbani. 2019. "Synthesis of a Novel Porphyrin-Based Metal–Organic Framework (Co-Por MOF)" Proceedings 41, no. 1: 83. https://doi.org/10.3390/ecsoc-23-06483
APA StyleTayebi, L., Rahimi, R., & Rabbani, M. (2019). Synthesis of a Novel Porphyrin-Based Metal–Organic Framework (Co-Por MOF). Proceedings, 41(1), 83. https://doi.org/10.3390/ecsoc-23-06483




