Abstract
We have developed a new idea to synthesize key intermediate molecule by utilizing deep eutectic solvent (DES) and ultrasound in a multistep reaction to ensure process cost-effective. Key intermediate (3) and final compounds (4a–n) were synthesized in a higher yield of 95% and 80–88% respectively. Further, final compounds (4a–n) were assessed for their anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation. The compounds 4f, 4g, 4j, 4l, and 4m showed good anti-inflammatory activity, while 4f, 4i, and 4n exhibited very good analgesic activity as compared to the standard drug. The ulcerogenicity of selected compounds was far less than the indomethacin. The ligands had also shown a good docking score (4f = −6.859 and 4n = −7.077) as compared to control indomethacin (−6.109). State-of-art DFT theory was used to validate the lipid peroxidation mechanism of the active compounds which was in good agreement with the variations of BDEs and IP of the tested compounds.
Keywords:
Thiazole-indole; DES; ultrasound; anti-inflammatory; analgesic; ulcerogenic; lipid peroxidation; molecular docking; DFT. 1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a profound application for the treatment of inflammatory diseases and pain. The NSAIDs are the choice of treatment in various inflammation and pain related problems such as osteoarthritis, rheumatoid arthritis, spondylitis and gout [1,2,3]. A mechanism based action of these drugs are exerted through the inhibition of cyclooxygenase type of enzymes, a principal enzyme which is used in the conversion of arachidonic acid to prostaglandin [4,5,6]. It has been reported that two forms of cyclooxygenase are involved in the pathogenesis of pain and inflammation, COX-1 and COX-2 [7,8]. However, their regulation and expression in the body are different [8,9] COX-1 is known constitutive enzyme which helps in cytoprotection in the gastrointestinal tract (GI). The inhibition COX-1 produces the undesired side effects of NSAIDs, for example, gastrointestinal toxicity because of their ulcerogenic effects. The COX-2 is an inducible enzyme that works through the mediation of the selective inflammatory signal and the therapeutic anti-inflammatory action of NSAIDs is produced by the inhibition of COX-2 [10,11,12,13,14]. Based on this observation, many selective COX-2 inhibitors like celecoxib, rofecoxib, and valdecoxib emerged as relatively safe NSAID'S together with improved gastric problems. However, the reporting of the cardiovascular side effects, for example, increased risk of myocardial infarction, stroke, heart failure and hypertension caused the withdrawal of many COX-2 inhibitors from the market [15]. This encouraged research professional to develop newer chemical entities as anti-inflammatory agents with minimal side effects.
Indole ring and its derivatives have emerged as privileged pharmacophore representing more than thousands natural isolates with known biological and pharmaceutical activities such as anti-inflammatory and analgesic activity [16,17,18,19], antimicrobial activity [20], antitumor activity [21] and anticonvulsant activity [22]. This ring is also a vital part of indomethacin, which is currently marketed as NSAIDs. However, the gastric safety profile of indomethacin is not promising and it produces gastrointestinal toxicity because of its ulcerogenic effects. In recent times, research reports highlighting the usefulness of the development of new coumarinylthiazoles as an anti-inflammatory agent and analgesic agents have also been published [23,24,25]. Thiazole and indole type of moieties were reported to synthesize by utilizing harsh chemicals/solvents which causes environmental pollution as well as raise the risk of health issues [26,27]. An alternative to such solvents such as deep eutectic solvent (DES) is the most valuable choice for varieties of organic transformations [28,29]. DES is usually a mixture of compounds having melting points less than their mixing components. The most versatile DES was prepared from choline chloride and some hydrogen bond donor (urea, glycerol) [29]. Depression in the melting point of DES is associated with molecular interaction of choline chloride and hydrogen bond donor part [29].
Immense application of ultrasound has been highlighted recently in organic and material science [30,31]. Ultrasound increased the rate of reaction by acoustic cavitation phenomena generated as a result of initiation, growth and collapse of bubbles during the course of reactions.
Keeping these things and with extended work [32,33,34,35] of our group to the development of new chemical templates in order to discover novel NSAIDs, authors planned to synthesize some molecules with a low budget and utilizing deep eutectic solvent and ultrasound technique to fulfill green chemistry approach.
2. Results and Discussion
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation as well as the experimental conclusions that can bedrawn.
2.1. Chemistry
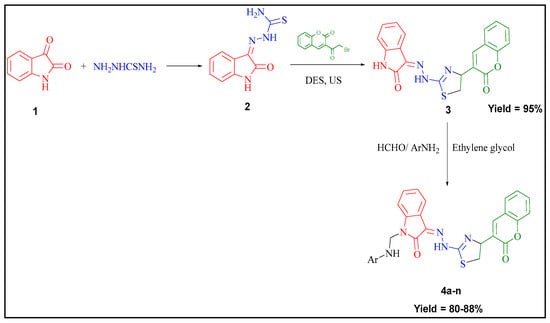
1-(Substituted phenylamino methyl)-3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl) hydrazono) indolin-2-ones were synthesized by treating 3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl)hydrazono )indolin-2-one (3) with substituted aromatic amines and formaldehyde in ethylene glycol as depicted in Figure 1. Prepared compounds were elucidated by FT-IR, 1H-NMR, 13C-NMR, mass and elemental analysis. In general, absorption bands due to two -NH group appeared in the IR spectra at around 3200 cm−1. Other bands due to -C=N and two -C=O functional groups were found at around 1600 cm−1 and 1700cm−1, respectively. In the 1H-NMR spectra, two -NH peak appeared at around 9 and 10 ppm. The lower value provides information as a singlet due to -NH attached as -CH2NH with indolinone nitrogen as a characteristic peak. Value at δ 5 ppm confirms the presence of -CH2 which is another important peak for identification. Further, characteristics peak of -CH2 of -CH2NH was confirmed by 13C-NMR around δ 69 ppm.
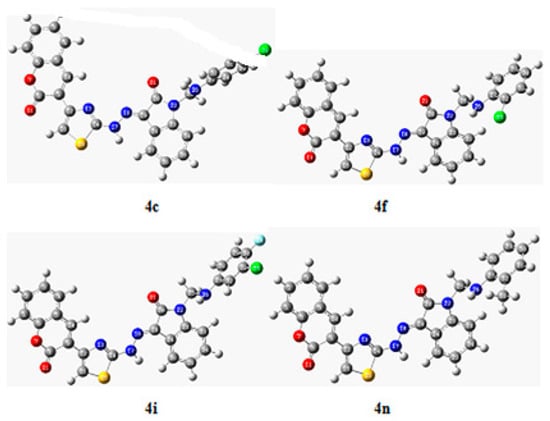
Figure 1.
The optimized structure with numbering of In-H synthesized derivatives.
The characterization data of all the synthesized compounds are provided below.
2-(2-Oxoindolin-3-ylidene)hydrazine carbothioamide (2): M.P.: 222–224°C; %Yield: 72; IR (KBr) cm−1: 3413, 3352 and 3216 (N-H), 1693 (C=O).1H-NMR (CDCl3, DMSO-d6) ppm: 6.72 (s, 1H, NH), 6.92 (d, J=12Hz, 1H, Ar-H), 7.03 (t, J=8Hz, 1H, Ar-H), 7.34 (t, J=8Hz, 1H, Ar-H), 8.04 (d, J=12Hz 1H, Ar-H), 9.99 (s, 1H, NH), 10.55 (s, 2H, NH); Elemental Analysis: Calcd. For (C9H8N4OS), Found % (Calculated %): C, 49.07 (49.08); H, 3.65 (3.66); N, 25.43 (25.44).
3-(2-(4-(2-Oxo-2H-chromen-3-yl)-4,5-dihydrothiazol-2-yl)hydrazono)indolin-2-one(3): M.P.: 240–242 °C; % Yield: 95; IR (KBr) cm−1: 1692 and 1703 (C=O), 3315 and 3253 (N-H), 1612 (C=N), 1543 (C=C).1H-NMR (CDCl3, DMSO-d6) ppm: 7.03 (t, J=8Hz, 1H, Ar-H), 7.39 (m, 8H, Ar-H, NH), 8.28 (s, 2H, Ar-H), 10.25 (s, 1H, -NH=N-); Elemental Analysis: Calcd. For (C20H12N4O3S), Found % (Calculated %): C, 61.84 (61.85); H, 3.10 (3.11); N, 14.42 (14.43).
3-{[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1-phenylaminomethyl-1,3-dihydro-indol-2-one (4a): M.P.: 245–247 °C; %Yield: 85; IR (KBr) cm−1: 1683 and 1710 (C=O), 3309 and 3251 (N-H), 1613 (C=N), 1546 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.13 (s, 2H, CH2), 7.44-8.10 (m, 13H, Ar-H), 9.35 (s, 1H, NH), 10.53 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 171.0 (C=N, thiazolidine),159 and 162 (2CO), 156 (1C, C=N),143.4, 140.9, 139.07, 139.0, 138.6, 131.0,129.8, 129.5, 127.8, 126.8, 125.5, 124.3,123.4, 121.2, 117.1, 112.4, (Ar-C), 69.3 (1C, CH2); Elemental Analysis: Calcd. For (C27H19N5O3S), Found % (Calculated %): C, 65.70 (65.71); H, 3.87 (3.88); N, 14.18 (14.19). Mass (m/z): 493 (M+, C27H19N5O3S), 200 (C11H6NOS), 175 (C10H7OS), 168 (C12H10N), 159 (C8H7N4), 132 (100%, C7H6N3), 106 (C7H8N).
1-[(4-Fluoro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4b): M.P.: 242–244 °C;%Yield: 82; IR (KBr) cm−1: 1683 and 1704 (C=O), 3312 and 3264 (N-H), 1613 (C=N), 1543 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.10 (s, 2H, CH2), 7.42 (m, 14H, Ar-H), 9.15 (s, 1H, NH), 10.55 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 172.0 (C=N, thiazolidine),160 and 161 (2CO), 155 (1C, C=N),145.2, 143.1, 140.2, 139.0, 138.4, 132.5,130.7, 129.3, 128.9, 127.6, 125.3,124.4, 122.2, 118.3, 114.3, (Ar-C), 68.9(1C, CH2); MS (m/z): 511 (M+), 513 (M++2); Elemental Analysis: Calcd. For (C27H18N5O3SF), Found % (Calculated %): C, 63.38 (63.40); H, 3.55 (3.55); N, 13.68 (13.69).
1-[(4-Chloro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4c): M.P.: 233–235 °C; %Yield: 85; IR (KBr) cm−1: 1689 and 1705 (C=O), 3309 and 3251 (N-H), 1613 (C=N), 1544 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.14 (s, 2H, CH2), 7.39 (m, 14H, Ar-H), 9.25 (s, 1H, NH), 10.50 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 170.0 (C=N, thiazolidine), 161 and 162 (2C, C=O), 157 (1C, C=N), 146.1, 143.1, 140.3, 139.1, 138.6, 133.5, 131.7, 129.2, 128.4, 127.7, 126.3, 125.2, 123.1, 117.9, 112.8, (Ar-C), 68.6(1C, CH2); MS(m/z): 528 (M+), 530 (M++2); Elemental Analysis: Calcd. For (C27H18N5O3SCl), Found % (Calculated %): C, 61.41 (61.42); H, 3.44 (3.44); N, 13.25 (13.26).
1-[(4-Bromo-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4d): M.P.:241–243 °C; %Yield: 80; IR (KBr) cm−1: 1685 and 1704 (C=O), 3313 and 3251 (N-H), 1611 (C=N), 1547 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.14 (s, 2H, CH2), 7.40 (m, 14H, Ar-H), 9.30 (s, 1H, NH), 10.51 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 171.0 (C=N, thiazolidine), 162 and 163 (2C, C=O), 156 (1C, C=N),145.5, 143.6, 140.3, 139.8, 138.3, 133.6,131.4, 129.7, 128.1, 127.7, 126.9, 123.6, 118.9, 112.9, (Ar-C), 69.3(1C, CH2); MS(m/z): 572 (M+), 574 (M++2); Elemental Analysis: Calcd. For (C27H18N5O3SBr), Found % (Calculated %): C, 56.64 (56.65); H, 3.16 (3.17); N, 12.22 (12.23).
1-[(2-Nitro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4e): M.P.: 244–246 °C; %Yield: 85; IR (KBr) cm−1: 1684 and 1705 (C=O), 3310 and 3255 (N-H), 1613 (C=N), 1543 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.07 (s, 2H, CH2), 7.42 (m, 14H, Ar-H), 9.15 (s, 1H, NH), 10.48 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 170.0 (C=N, thiazolidine), 163 and 164 (2CO), 157 (1C, C=N), 146.4, 143.8, 141.6, 140.4, 139.7, 135.6, 133.4, 130.7, 128.5, 127.5, 126.4, 124.6, 118.6, 112.3, (Ar-C), 70.1(1C, CH2); MS (m/z): 538 (M+), 540 (M++2); Elemental Analysis: Calcd. For (C27H18N6O5S), Found % (Calculated %): C, 60.21 (60.22); H, 3.36 (3.37); N, 15.60 (15.61).
1-[(2-chloro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4f): M.P.: 239–241 °C; %Yield: 83; IR (KBr) cm−1: 1689 and 1707 (C=O), 3311 and 3252 (N-H), 1613 (C=N), 1545 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.10 (s, 2H, CH2), 7.36 (m, 14H, Ar-H), 9.36 (s, 1H, NH), 10.55 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 172.0 (C=N, thiazolidine), 161 and 162 (2CO), 157 (1C, C=N), 146.5, 143.9, 140.8, 140.1, 138.7, 135.4, 133.7, 129.7, 128.8, 127.6, 126.3, 124.7, 119.3, 114.1, (Ar-C), 68.3(1C, CH2); Elemental Analysis: Calcd. For (C27H18N5O3SCl), Found % (Calculated %): C, 61.41 (61.42); H, 3.43 (3.44); N, 13.25 (13.26). Mass (m/z): 527 (M+, C27H18N5O3SCl), 528 (M++1), 202 (C12H9NCl), 175 (C10H7OS), 132 (100%, C7H6N3), 111 (C6H4Cl), 59 (C2H3S).
1-[(2,4-Dinitro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4g): M.P.: 247–249 °C; %Yield: 80; IR (KBr) cm−1: 1686 and 1704 (C=O), 3292 and 3252 (N-H), 1612 (C=N), 1544 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.15 (s, 2H, CH2), 7.45 (m, 13H, Ar-H), 9.33 (s, 1H, NH), 10.51 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 169.0 (C=N, thiazolidine), 159 and 161 (2CO), 155 (1C, C=N), 145.7, 142.5, 140.2, 139.1, 137.4, 135.7, 133.5, 129.6, 128.6, 127.3, 124.7, 118.9, 112.3, (Ar-C), 69.7(1C, CH2); MS(m/z): 583 (M+), 585 (M++2); Elemental Analysis: Calcd.For(C27H17N7O7S), Found % (Calculated %): C, 55.56 (55.57); H, 2.94 (2.94); N, 16.79 (16.80).
3-{[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1-([1,2,4]triazol-4-ylaminomethyl)-1,3-dihydro-indol-2-one (4h): M.P.: 236–238 °C; %Yield: 80; IR (KBr) cm−1: 1685 and 1706 (C=O), 3253 and 3279 (N-H), 1613 (C=N), 1543 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.17 (s, 2H, CH2), 7.48 (m, 12H, Ar-H), 9.42 (s, 1H, NH), 10.47 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 170.0 (C=N, thiazolidine), 161 and 161 (2CO), 157 (1C, C=N), 144.8, 140.2, 137.4, 135.9, 132.4, 129.4, 128.7, 127.5, 124.3, 116.9, 112.3, (Ar-C), 70.2(1C, CH2); MS(m/z): 484 (M+), 486 (M++2); Elemental Analysis: Calcd. For (C23H16N8O3S), Found % (Calculated %): C, 57.01 (57.02); H, 3.32 (3.33); N, 23.12 (23.13).
1-[(3-Chloro-4-fluoro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4i): M.P.: 246–248 °C; %Yield: 85; IR (KBr) cm−1: 1684 and 1703 (C=O), 3240 and 3273 (N-H), 1612 (C=N), 1544 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.05 (s, 2H, CH2), 7.41 (m, 13H, Ar-H), 9.31 (s, 1H, NH), 10.55 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 171.0 (C=N, thiazolidine), 161 and 163 (2CO), 156 (1C, C=N), 146.9, 143.4, 140.6, 139.2, 137.6, 135.7, 133.8, 129.3, 128.3, 127.6, 124.2, 117.4, 112.3, (Ar-C), 68.6(1C, CH2); MS(m/z): 546 (M+), 548 (M++2); Elemental Analysis: Calcd. For (C27H17N5O3SClF), Found % (Calculated %): C, 59.38 (59.40); H, 3.14 (3.14); N, 12.82 (12.83).
3-{[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1-(pyridine-4-ylaminomethyl)-1,3-dihydro-indol-2-one (4j):M.P.: 237–239 °C; %Yield: 88; IR (KBr) cm−1: 1687 and 1705 (C=O), 3244 and 3268 (N-H), 1613 (C=N), 1545 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.17 (s, 2H, CH2), 7.45 (m, 14H, Ar-H), 9.38 (s, 1H, NH), 10.57 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 172.0 (C=N, thiazolidine), 161 and 163 (2CO), 156 (1C, C=N),144.8, 143.5, 140.4, 139.0, 138.3, 132.5,130.9, 129.3, 128.8, 127.6, 125.1,124.7, 122.9, 116.9, 112.6, (Ar-C), 68.3(1C, CH2); MS (m/z): 594 (M+), 596 (M++2); Elemental Analysis: Calcd. For (C26H18N6O3S), Found % (Calculated %): C, 63.14 (63.15); H, 3.66 (3.67); N, 16.98 (16.99).
3-{[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1-(pyridine-3-ylaminomethyl)-1,3-dihydro-indol-2-one (4k):M.P.: 231–233 °C; %Yield: 85; IR (KBr) cm−1: 1686 and 1706 (C=O), 3251 and 3277 (N-H), 1612 (C=N), 1543 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.15 (s, 2H, CH2), 7.47 (m, 14H, Ar-H), 9.35 (s, 1H, NH), 10.55 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 172.0 (C=N, thiazolidine),161 and 162 (2CO), 156 (1C, C=N),144.9, 143.6, 140.2, 139.0, 138.5, 132.3,130.7, 129.5, 128.7, 127.3, 125.5,124.9, 122.8, 116.6, 112.4, (Ar-C), 68.3(1C, CH2); MS (m/z): 594 (M+), 596 (M++2); Elemental Analysis: Calcd.For(C26H18N6O3S), Found % (Calculated %): C, 63.14 (63.15); H, 3.66 (3.67); N, 16.98 (16.99).
1-[(4-Nitro-phenylamino)-methyl]-3-{[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1,3-dihydro-indol-2-one (4l):M.P.: 241–243 °C; %Yield: 90; IR (KBr) cm−1: 1684 and 1702 (C=O), 3255 and 3278 (N-H), 1612 (C=N), 1544 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 5.18 (s, 2H, CH2), 7.46 (m, 14H, Ar-H), 9.32 (s, 1H, NH), 10.54 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 170.0 (C=N, thiazolidine), 163 and 164 (2CO), 158 (1C, C=N), 146.6, 143.7, 141.9, 140.5, 139.7, 135.2, 133.5, 130.1, 128.2, 127.7, 126.1, 124.3, 116.9, 112.3, (Ar-C), 70.2(1C, CH2); MS (m/z): 538 (M+), 540 (M++2); Elemental Analysis: Calcd. For (C27H18N6O5S), Found % (Calculated %): C, 60.21 (60.22); H, 3.36 (3.37); N, 15.60 (15.61).
3-{[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1-(p-tolylamino-methyl)-1,3-dihydro-indol-2-one (4m): M.P.: 244–246 °C; %Yield: 84; IR (KBr) cm−1: 1685 and 1706 (C=O), 3252 and 3273 (N-H), 1608 (C=N), 1544 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 2.23 (s, 3H, CH3), 5.11 (s, 2H, CH2), 7.41 (m, 14H, Ar-H), 9.31 (s, 1H, NH), 10.48 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 172.0 (C=N, thiazolidine), 160 and 163 (2CO), 156 (1C, C=N), 146.8, 144.2, 142.4, 140.6, 139.9, 135.3, 133.8, 130.5, 128.8, 127.1, 126.3, 124.8, 117.4, 114.1, (Ar-C), 70.3(1C, CH2); MS (m/z): 507 (M+), 509 (M++2); Elemental Analysis: Calcd. For (C28H21N5O3S), Found % (Calculated %): C, 66.25 (66.26); H, 4.16 (4.17); N, 13.79 (13.80).
3-{[4-(2-Oxo-2H-chromen-3-yl)-thiazol-2-yl]-hydrazono}-1-(o-tolylamino-methyl)-1,3-dihydro-indol-2-one (4n):M.P.: 237–239 °C; %Yield: 86; IR (KBr) cm−1: 1686 and 1703 (C=O), 3251 and 3282 (N-H), 1612 (C=N), 1543 (C=C); 1H-NMR (CDCl3, DMSO-d6) ppm: 2.21 (s, 3H, CH3), 5.13 (s, 2H, CH2), 7.43 (m, 14H, Ar-H), 9.34 (s, 1H, NH), 10.53 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6); 171.0 (C=N, thiazolidine), 161 and 162 (2CO), 157 (1C, C=N), 145.9, 144.2, 142.6, 140.3, 139.2, 135.6, 133.5, 130.2, 128.9, 127.6, 126.1, 124.9, 118.2, 112.3, (Ar-C), 68.3(1C, CH2); Elemental Analysis: Calcd. For (C28H21N5O3S), Found % (Calculated %): C, 66.25 (66.26); H, 4.16 (4.17); N, 13.80 (13.80); Mass (m/z): 507 (M+, C28H21N5O3S), 387 (C20H11N4O3S), 200 (C11H6NOS), 132 (100%, C7H6N3), 120 (C8H10N), 90 (C6H4N), 59 (C2H3S).
2.1.1. Significance of DES and Ultrasound Blend of Techniques to the Synthesis of Key Intermediate 3-(2-(4-(2-Oxochroman-3-Yl) Thiazol-2-Yl) Hydrazono) Indolin-2-One
To develop the efficient method as compared to conventional, we have conducted the synthesis of key intermediate (3) utilizing biocompatible deep eutectic solvent (DES) and ultrasound blend of technique. As a result of combined use of DES and ultrasound, there have been found an increase in % yield of key intermediate as high as 95% with the expense of 1hr only. Whereas, a similar type of organic transformations using dioxane and another organic solvent together with conventional heating were reported to have % yield around 44–68% in 3–4 hrs. [36,37,38]. Further, we have also found 80–88% of all final compounds (4a–n) utilizing ultrasound as a source of heating. Some of our earlier work and other related literature also mentioned the significance DES and ultrasound technology as an energy saving process [29,39,40] which is certainly a good favor of our present work.
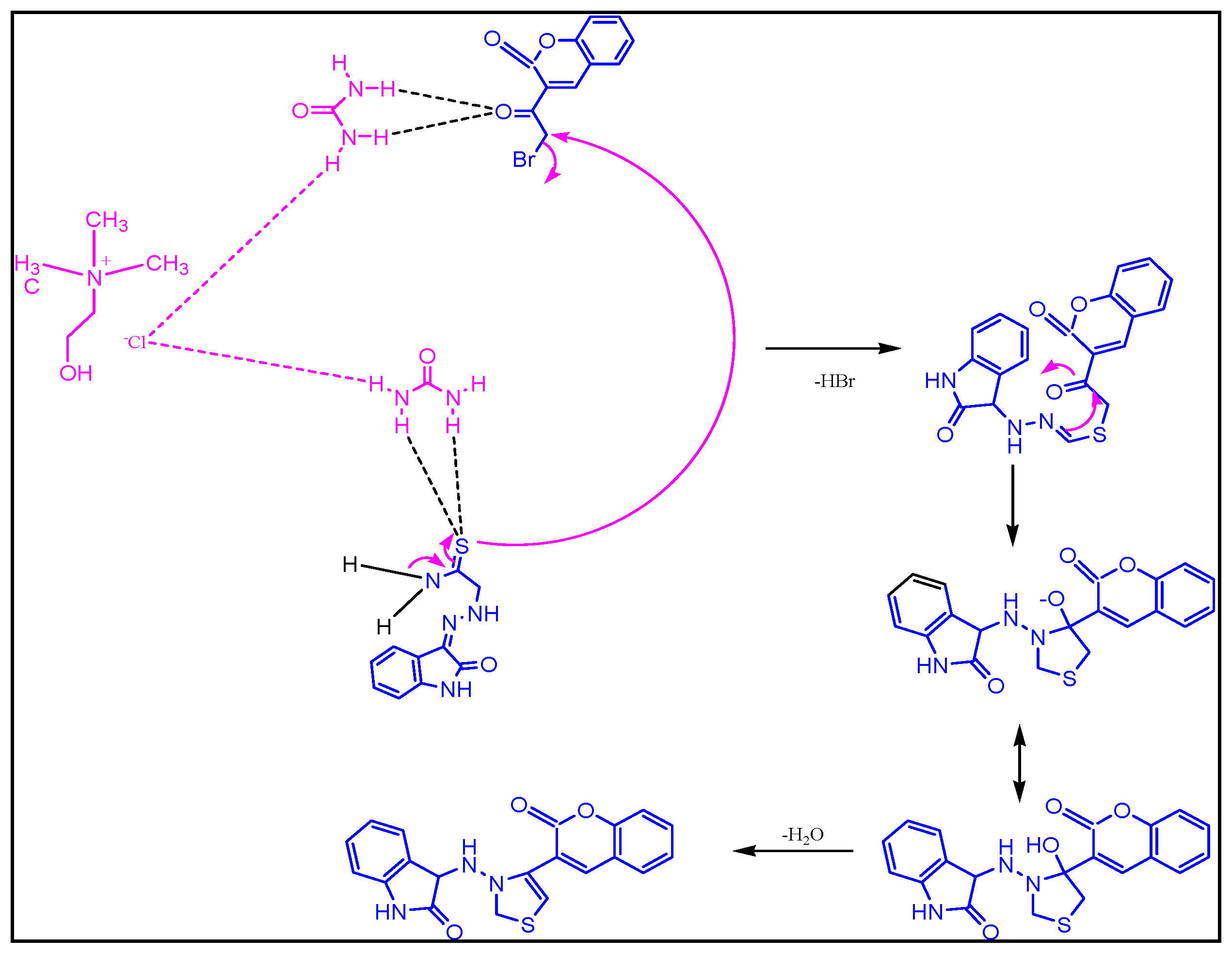
2.1.2. Plausible Mechanism Involved to the Formation of Key Intermediate,3-(2-(4-(2-Oxochroman-3-Yl) Thiazol-2-Yl) Hydrazono) Indolin-2-One
The exact mechanism of formation of the desired intermediate compound is not yet clear. But it was suggested by some researchers that urea part of DES (Choline chloride: urea, 1:2) catalyze the reaction by making hydrogen bond. Thus, urea in deep eutectic solvent involved to stabilize the acetyl moiety of 3-bromoacetylcoumarin via hydrogen bonding, which was further attacked by amide functional group of hydrazine thioamide to form key intermediate, 3-(2-(4-(2-oxochroman-3-yl)thiazol-2-yl)hydrazono) indolin-2-one through cyclization and dehydration process (Scheme 1).
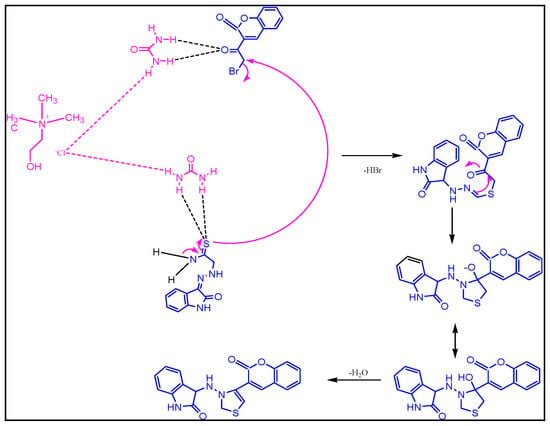
Scheme 1.
Proposed mechanism involved to the formation of key intermediate, 3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl) hydrazono) indolin-2-one using DES.
Moreover, ultrasound also played a significant role in the formation of the desired compound. Under the influence of sonic waves inside the reaction vessel, there was the formation of microscopic bubbles, as a result of high temperature and pressure [28,29,30,31]. These tiny microscopic bubbles also help in the cyclization process.
2.2. Biology
2.2.1. Anti-Inflammatory Activity
Anti-inflammatory activity of the synthesized compounds (4a–n) was evaluated by carrageenan-induced paw edema method. An oral dose of 10mg/kg was used for compounds and compared with the standard. Anti-inflammatory activity was accessed through percentage inhibition after 2 h and 4 h (Table 1).

Table 1.
Anti-inflammatory activity of 1-(Substituted phenyl amino methyl)-3-(2-(4-(2-oxo chroman-3-yl) thiazol-2-yl) hydrazono) indolin-2-one (4a–n).
Anti-inflammatory activity in terms of percentage inhibition for the test compounds are ranging from 5.57% to 80.94 % (Table 1), whereas standard drug showed 82.05% after 4 hours. Compounds 4f (72.42%), 4g (77.94%), 4j (77.90%), 4k (70.75%), 4l (80.94%) and 4m (78.42%) showed comparable results against the standard drug.
The structure of 1-(Substituted phenyl amino methyl)-3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl) hydrazono) indolin-2-one derivatives revealed that the compound 4l (Ar = 4-nitrophenyl) exhibited highest anti-anti-inflammatory activity. Other compounds of the series, namely, 4f (Ar = 2-chlorophenyl), 4g (Ar = 2,4-dinitrophenyl), 4j (Ar = 4-pyridyl), 4k (Ar = 2-pyridyl) and 4m (Ar = 4-methyl phenyl) also displayed significant anti-inflammatory activity. Two compounds, 4a (Ar = phenyl) and 4d (Ar = 4-bromophenyl) displayed negligible anti-inflammatory activity. All other compounds displayed moderate anti-inflammatory activity. Further, the number and position of substituents also count the variation in anti-inflammatory activity. Nitrogen bearing compounds 4g (Ar = 2,4-dinitrophenyl) and 4l (Ar = 4-nitrophenyl) showed highest anti-inflammatory activity. When chloro substituent present on ortho-position(4f) of phenyl ring displayed almost double activity as compared to a compound bearing parachloro compound (4c). Similarly, the difference in anti-inflammatory activity was found in compounds 4j & 4k and 4m & 4n due to different arrangements of substituents on the phenyl ring.
2.2.2. Analgesic Activity
Compounds under investigation showed analgesic activity ranging from 7.96% to 69.36% with reference drug of 73.61% (Table 2).

Table 2.
Analgesic activity of 1-(Substituted phenyl amino methyl)-3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl) hydrazono)indolin-2-one (4a–n).
All the tested compounds and standard drug are evaluated at 10mg/kg oral dose. It was identified that compound (4l) showed maximum anti-inflammatory activity produces least analgesic activity, but some selected compounds like- 4f, 4i and 4n displayed analgesic activity in a similar fashion as anti-inflammatory activity (Table 1, Table 2). Compound (4k) exhibited the least analgesic activity was among the top-ranked anti-inflammatory activity. On the contrary, many of compounds exhibited good analgesic properties were not displayed good anti-inflammatory activity and vice-versa (Table 1, Table 2).
After a close understanding of anti-inflammatory and analgesic potentials of compounds under present series, we have made a structure-activity relationship. Compounds possessing a substituted phenyl ring showed better anti-inflammatory and analgesic activity than a compound having an unsubstituted phenyl ring. In most of the cases, the substitution of electron withdrawing groups at C-2 and C-4 positions of phenyl ring resulted in potent compounds except compound 4d (Ar = 4-bromophenyl) that showed negligible anti-inflammatory activity. Compound 4i possessing two electron withdrawing groups exhibited moderate anti-inflammatory activity but good analgesic activity. Compound (4m) having an electron releasing group (-CH3) at C-4 position exhibited better anti-inflammatory activity but less analgesic activity. On the other hand, a methyl group at C-2 showed better anti-inflammatory and analgesic agent (4n). A steep decrease in analgesic activity was observed when the phenyl ring was replaced by a triazole ring (4h).
2.2.3. Acute Ulcerogenicity
Four compounds, namely, 4c (Ar = 4-chlorophenyl), 4f (Ar = 2-chlorophenyl), 4i (Ar = 4-fluoro-3-chlorophenyl) and 4n (Ar = 2-methylphenyl) were selected for their ulcerogenic activity. This selection was based on their anti-inflammatory and analgesic activity. Compounds were evaluated at oral dose of 30mg/kg relative to 10mg/kg indomethacin.
The ulcerogenic activity of these compounds revealed that all the compounds showed a lesser severity index for ulcerogenicity than indomethacin (Table 3). Compound 4n exhibited the highest severity index of 0.833 but it was only 20% of the severity shown by the standard. Mainly compounds, 4f, 4i and 4n displayed excellent anti-inflammatory, an analgesic with reduced ulcerogenic potential. Significant reduction in ulcerogenecity is ranging from 0.500 ± 0.129 to 0.833 ± 0.210, whereas standard drug indomethacin showed a high severity index of 4.500 ± 0.316.

Table 3.
Ulcerogenic activity and lipid peroxidation of 1-(Substituted phenyl amino methyl)-3-(2-(4-(2- oxochroman-3-yl) thiazol-2- yl) hydrazono)indolin-2-one.
2.2.4. Lipid Peroxidation
Gastrointestinal (GI) ulceration, bleeding and renal problems are common complications of NSAID'S consumption, which is directly related to lipid peroxidation. It has been evidenced that drug having less ulcerogenecity showed reduced malondialdehyde (a byproduct of lipid peroxidation) content [4,41]. We have examined the lipid peroxidation (LP) of compounds which exhibited maximum anti-inflammatory and analgesic activities (4c, 4f, 4i, 4n). It was measured as nmol of MDA/100mg of gastric tissue. We have found lipid peroxidation value maximum 6.71 ± 0.18 for indomethacin, whereas 3.89 ± 0.17, 4.08 ± 0.22, 4.26 ± 0.12 and 4.81 ± 0.13 for compounds 4i, 4c, 4f and 4n respectively. It was interesting to mention that all these compounds having electron withdrawing functionality on the phenyl ring (except 4n) exhibited less ulcerogenecity with reduced lipid peroxidation (Table 3).
2.2.5. DFT Results
As mentioned above, only the synthesized derivatives (In-H) that exhibited maximum anti-inflammatory and analgesic activities derivatives are subjected to lipid peroxidation (LP) test (Table 4, Figure 1). The tested In-H derivatives show the ability to scavenge LOO• free radical. To shed light on the small observed lipid peroxidation inhibition of In-H derivatives, bond dissociation enthalpies of the of i-NH function groups and ionization potential energies of the tested compounds and were calculated at the B3P86/6-311+G(d,p) level of theory (Table 4 and Figure 2).

Table 4.
BDEs (kcal/mol) of i-NH groups of the In-H synthesized derivatives and its corresponding ionization potential energies calculated at the B3P86/6-31+G(d,p) level of theory.
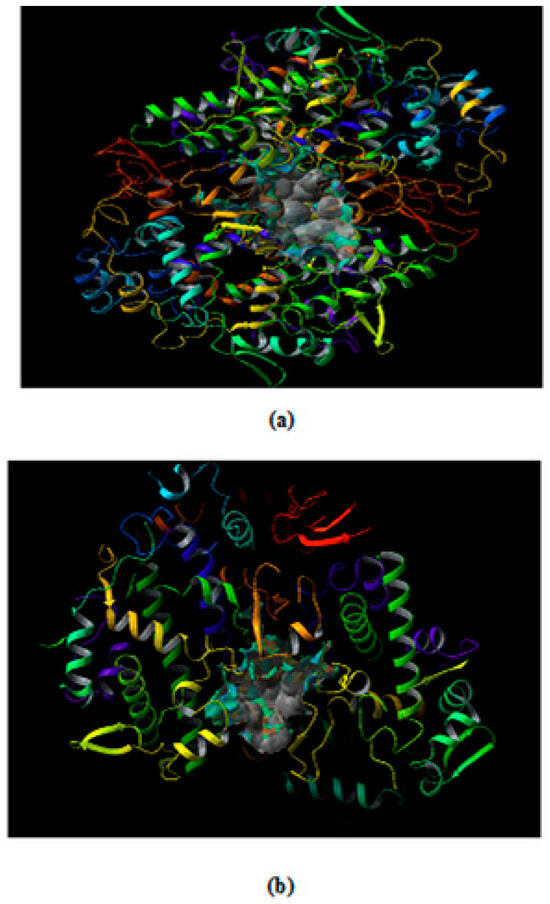
Figure 2.
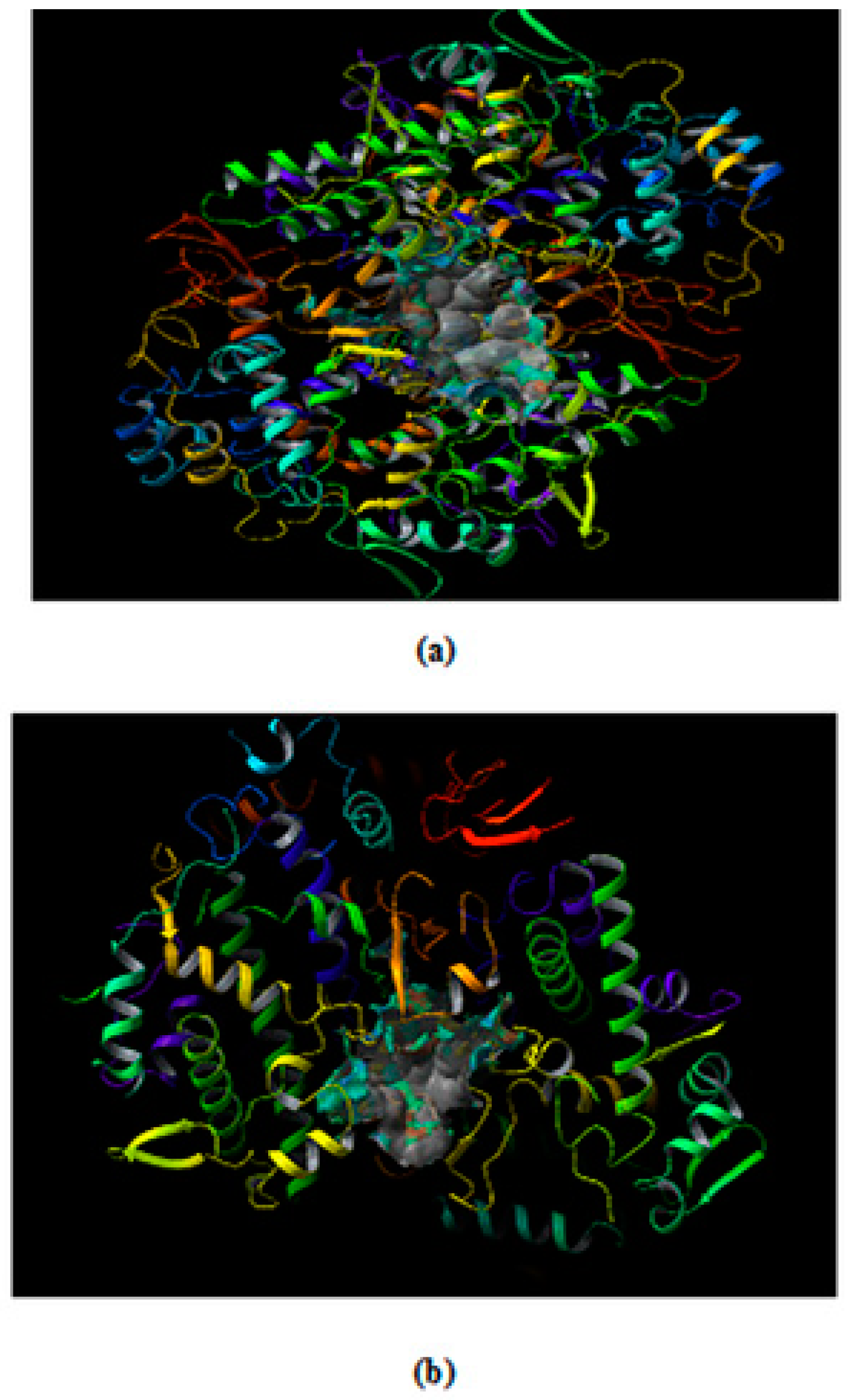
The binding site predicted where ligand is docked in COX-2 from (a) mouse (PDB ID 3NT1) (b) human (PDB ID: 5F19).
The tested compounds showed similar lipid peroxidation with a small variation between their values. This result is confirmed by the small differences of BDEs of the active 17-NH group and IP energies, where the maximum variations of BDEs and IPs are of 0.03 kcal/mol and 0.08 eV, respectively.
2.2.6. In-SilicoStudy
2.2.6.1. Target Protein Selection and Retrieval
The target protein COX-2 from two different organisms i.e. mouse and human are retrieved from protein data bank having PDB id 3NT1 and 5F91 respectively [42,43].
2.2.6.2. Protein (COX-2) Preparation and Validation
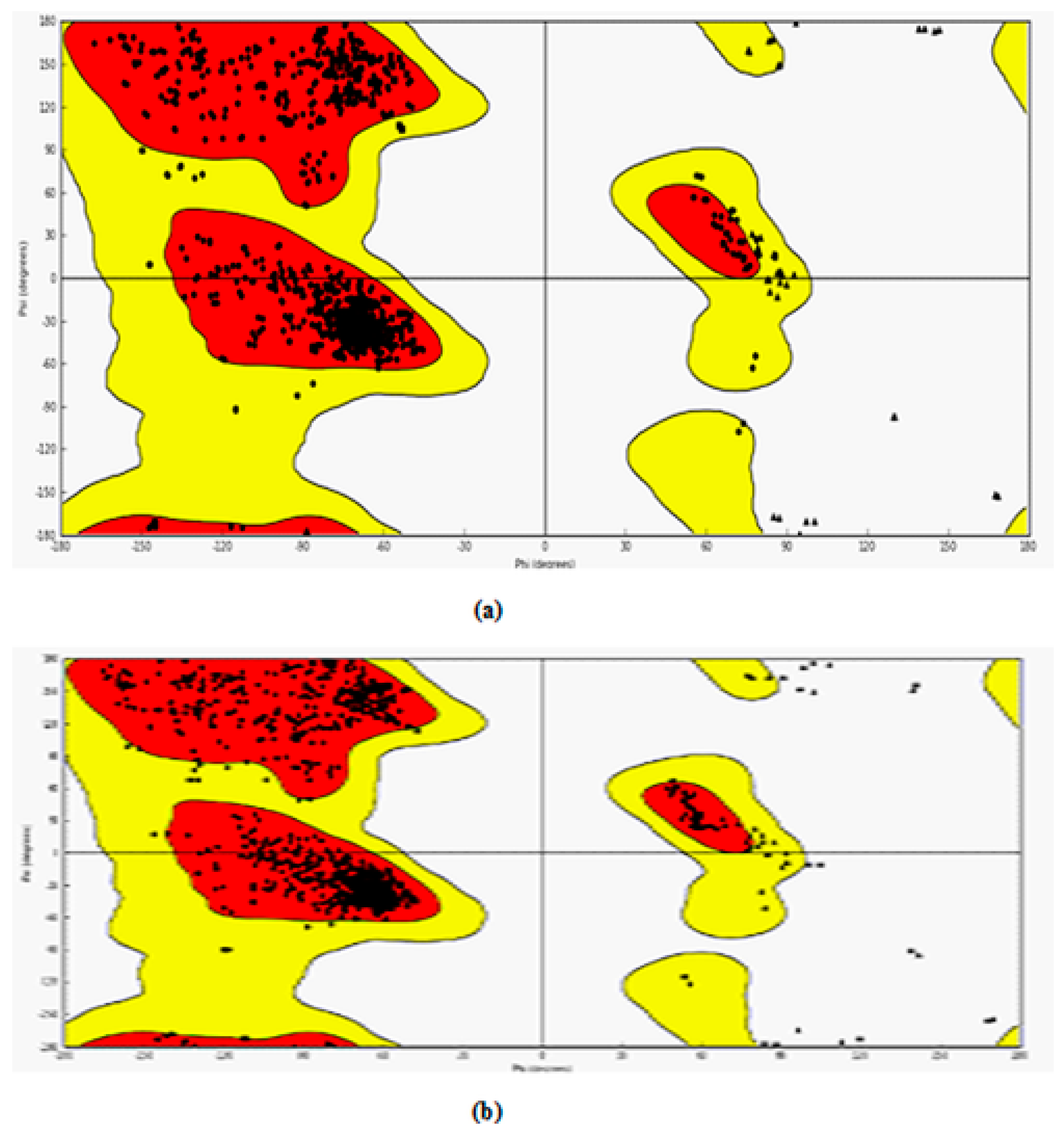
The protein structures obtained from PDB were modified suitably for the docking studies. The modified protein structures were validated through the Ramachandran plot. The Ramachandran plot of these two-target protein is shown in Figure 3a,b.
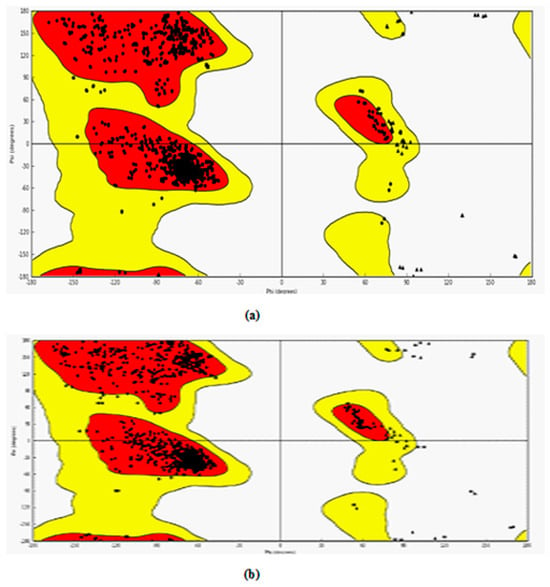
Figure 3.
The binding site predicted where ligand is docked in COX-2 from (a) mouse (PDB ID 3NT1) (b) human (PDB ID: 5F19).
The evaluation of phi/psi angles validates the protein structure as most of the residues are in the most favored region. In case of 3NT1, 90.8% amino acid residues are in the most favored regions whereas 8.8%, 0.1%, and 0.3% are in additional allowed regions, generously allowed regions and disallowed regions respectively. Similarly, in 5F91 90.7%, 9.1%, 0.1%, and 0.1% amino acid residues are in the most favored regions, additionally allowed regions, generously allowed regions and disallowed regions respectively. The result obtained allows the use of these structures for further docking studies.
2.2.6.3. Prediction and Evaluation of the Binding Site in COX-2
The Site map application predicts five different drug binding sites in both the target proteins. The site score for the protein 3NT1 were 1.078, 1.053, 1.048, 1.034 and 0.991. Similarly, the site score obtained for the protein 5F19 was 1.082, 1.051, 1.046, 1.034 and 0.990. As a rule of thumb binding site having a score above are considered as druggable pockets. In the present in-silico study site with the highest score were selected for the docking studies. The druggable pocket inside the respective target proteins is shown in Figure 2a 3NT1 and Figure 2b 5F19.
2.2.6.4. Ligand Preparation
The lowest energy conformation of each test ligands (4a–4n) was prepared for the docking studies as per the standard guidelines and used in the molecular docking studies.
2.2.6.5. Grid Generation in the Target Protein COX-2
After the determination of the exact location of the drug binding site in each target, protein grid was generated around the binding sites to specify the volume and location of the druggable pocket.
2.2.6.6. Molecular Docking Studies
Table 5. Summary of molecular docking score of different ligands against Cox-2 (target protein) from mouse (3NT1) and human (5F19).

Table 5.
Summary of molecular docking score of different ligands against Cox-2 (target protein) from mouse (3NT1) and human (5F19).
Present series (4a–n) undergo docking studies using Glide (version 7.0, Schrödinger, New York, USA) application of the Schrodinger Maestro interface. All the derivatives of 1-(substituted phenyl aminomethyl)-3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl) hydrazono) indolin-2-ones were docked to the active site of the target enzyme COX-2 (PDB ID: 3NT1 and 5F19). These compounds were compared with the reference drug (Indomethacin), considering docking score, E-model score and binding energy against mouse (3NT1) and human (5F19) model (Table 5).
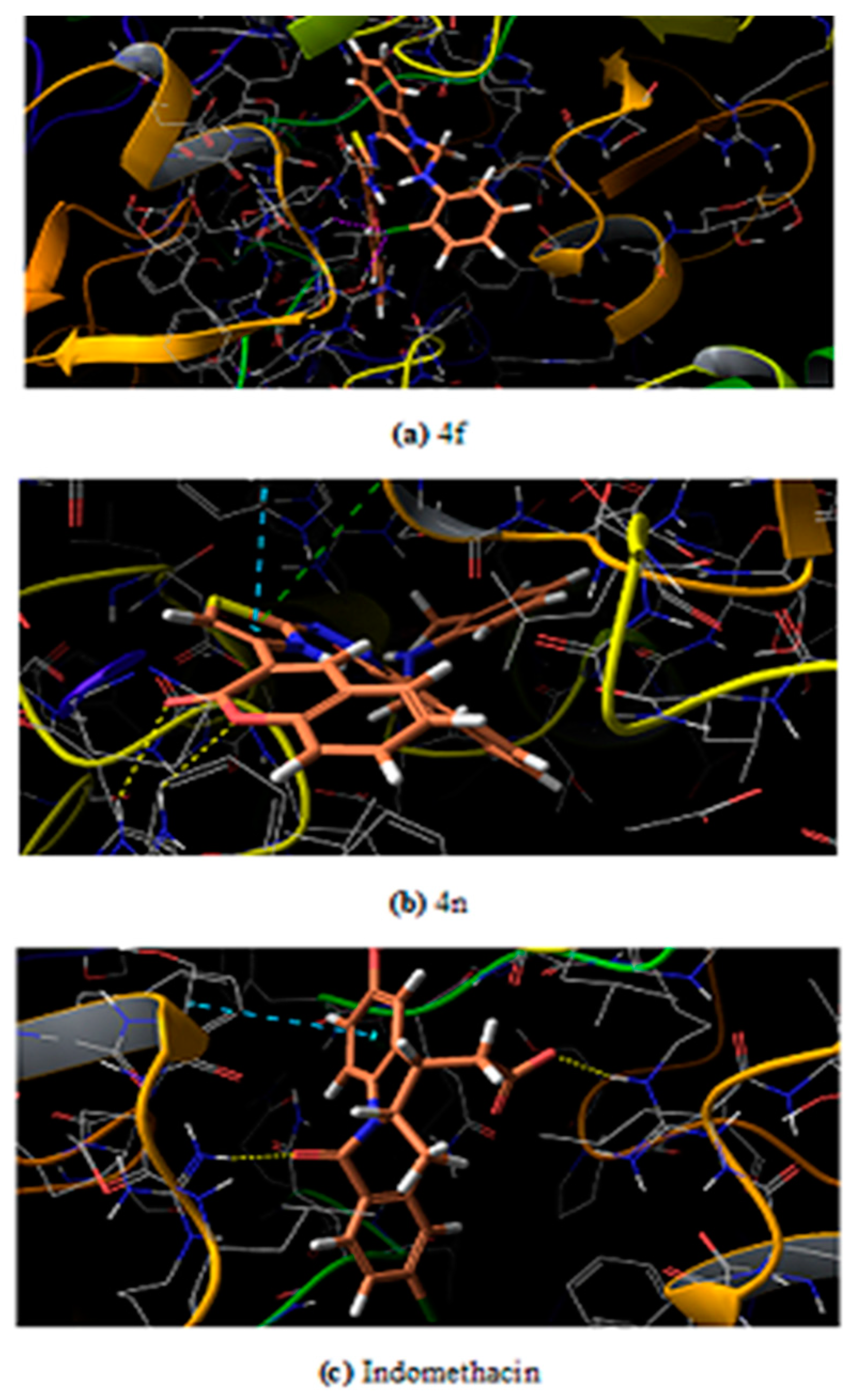
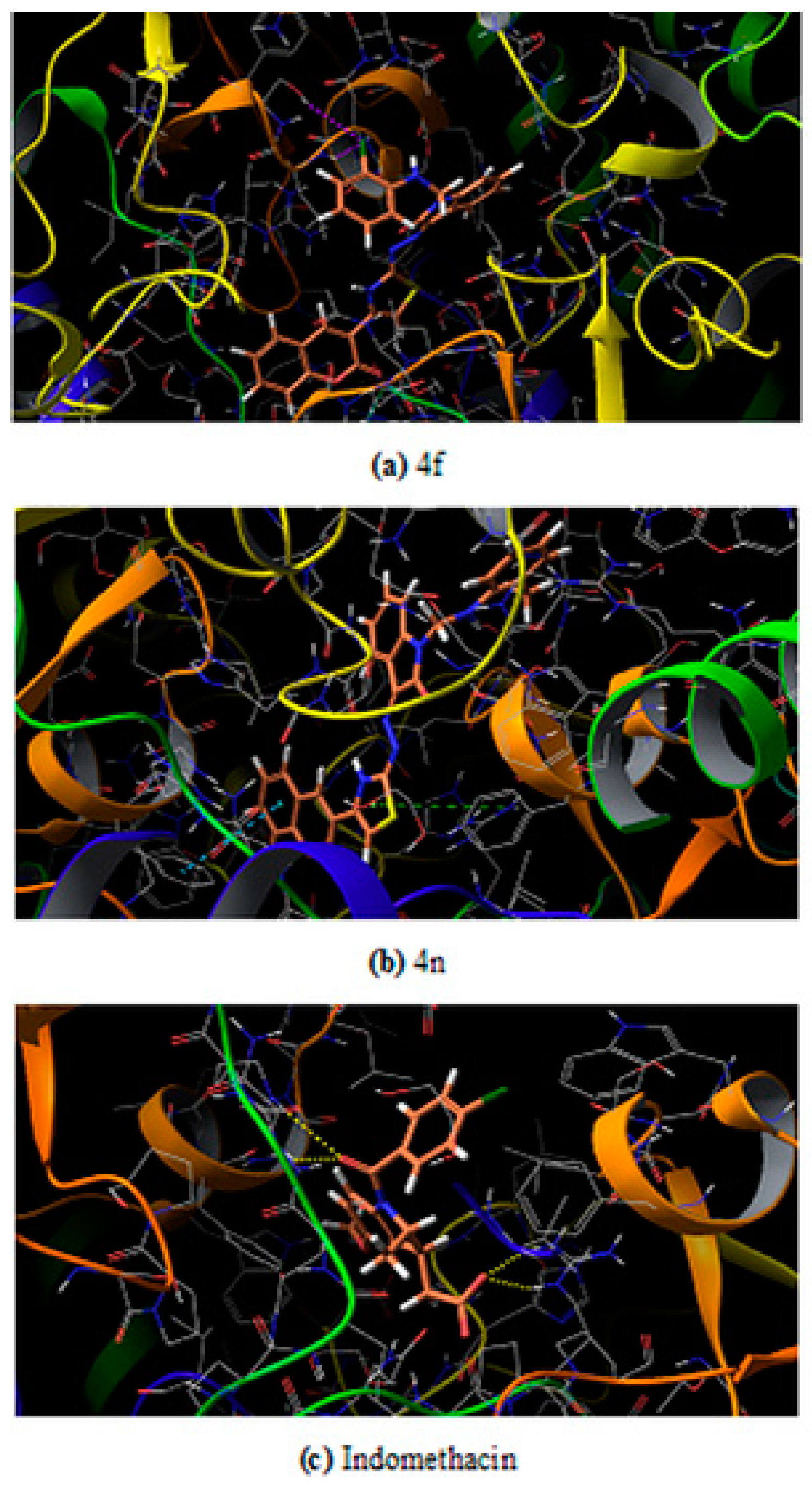
The maximum test ligands that are 4 a, b, c, e, g, h, i, j, k, l, m, n showed docking score lower than the control/reference drug (−6.324) against the mouse target protein. A similar pattern of docking score is observed against human target protein where all test ligands 4 a-n have lower docking score as compared to control having a score −6.109. It was believed that low binding energy dock conformer exhibited maximum stability. The two best compound on the basis of experimental results that are 4n and 4f have docking score −7.077 and −6.859 against human target protein respectively. The same two ligand 4n and 4f have a score −6.693 and −6.071 against mouse target protein respectively. The docked ligands (4n and 4f) inside the binding pocket of the respective target proteins (3NT1 and 5F19) is shown in Figure 4 and Figure 5.
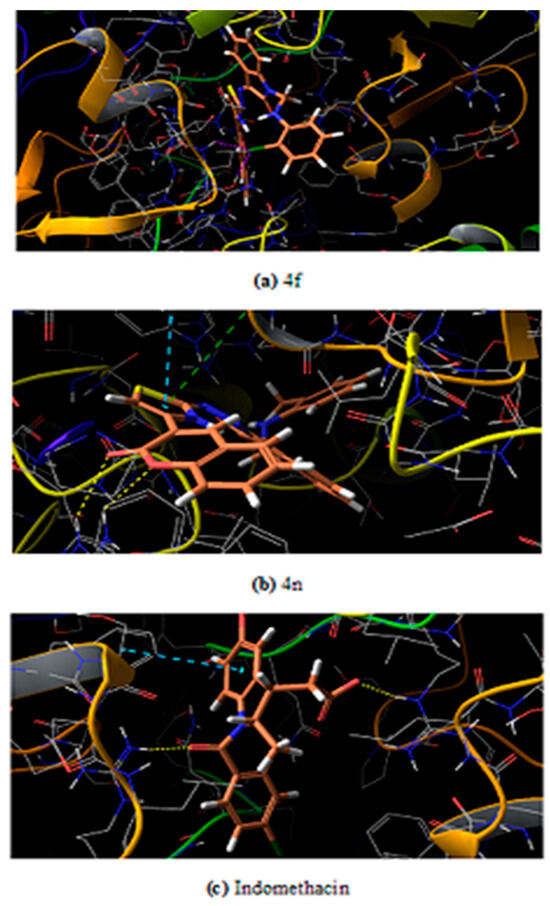
Figure 4.
Ligand inside the binding pocket of COX-2 from mouse (a) 4f (b) 4n (c) Indomethacin.
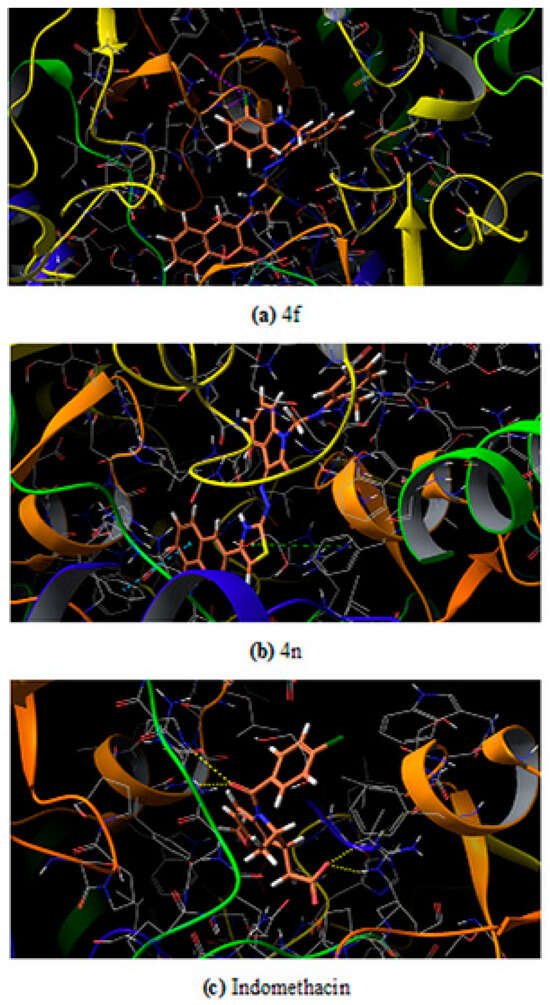
Figure 5.
Docked ligand inside from the binding pocket of COX-2 from human (a) 4f (b) 4n (c) Indomethacin.
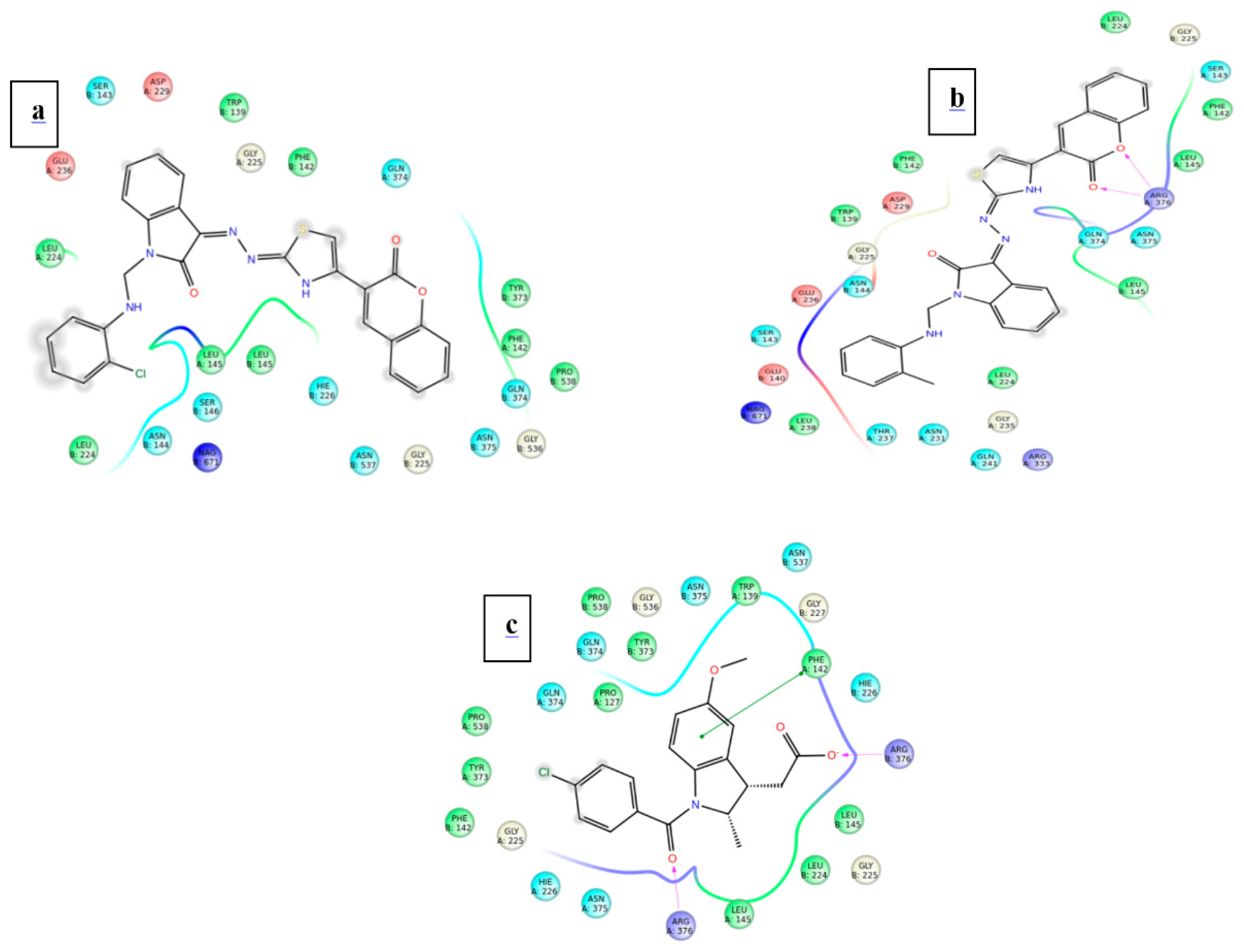
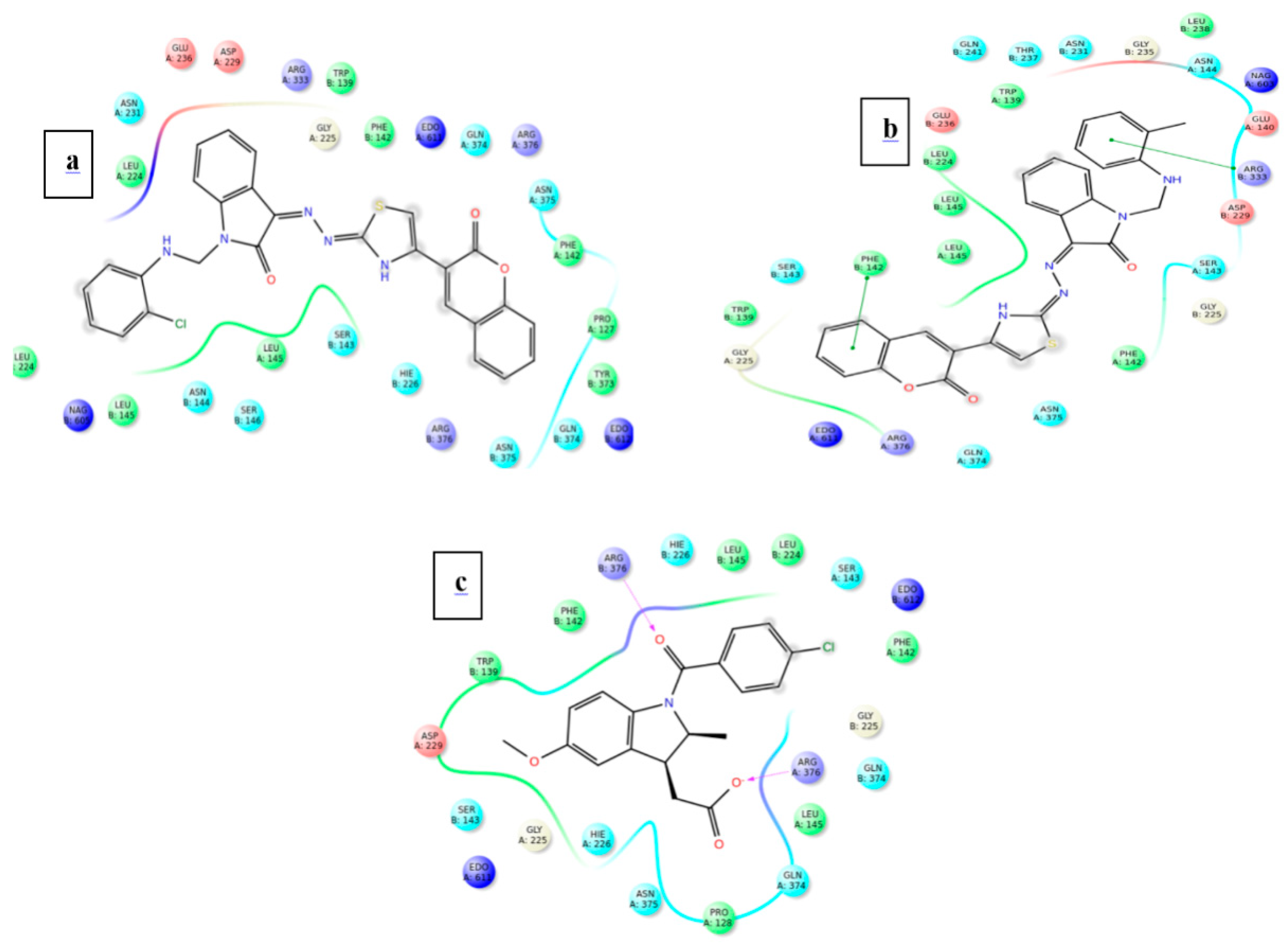
The further efficacy of the docking is interpreted in the terms interaction that exists between the ligand and the surrounding amino acid residues inside the druggable pocket. The overall binding interaction (in terms of bonding) for each ligand is summarized in Table 6 and Table 7 for the proteins 3NT1 and 5F19 respectively.

Table 6.
Types of interaction and amino acid residues involve in that interaction inside the binding pocket of Cox-2 from mouse (4NT1).

Table 7.
Types of interaction and amino acid residues involve in that interaction inside the binding pocket of Cox-2 from mouse (5F19).
Among the two potent ligands, 4n is more suitable for drug candidate as it possesses a strong affinity towards the target proteins. In 3NT1 is forms two hydrogen bonds with Arg 376, whereas in 5F19 two pi-pi stacking exists with the involvement of Phe 142 and Arg 333. In the case of 4f, there is no hydrogen bonding or pi-pi interaction is observed whether it is 3NT1 or 5F19. All these interactions are shown in Figure 6 and Figure 7 as ligand interaction diagram.
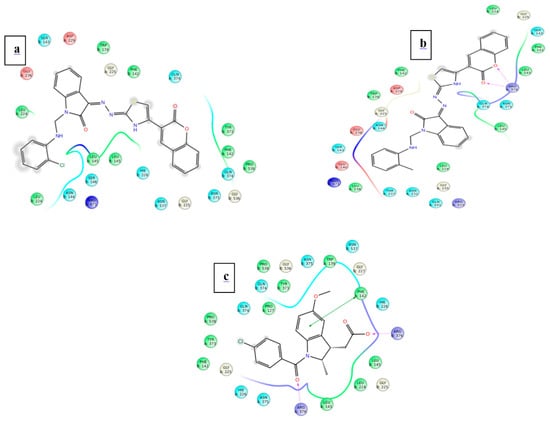
Figure 6.
Ligand interaction of test ligand with the target protein COX-2 from mouse (a) 4f (b) 4n (c) Indomethacin.
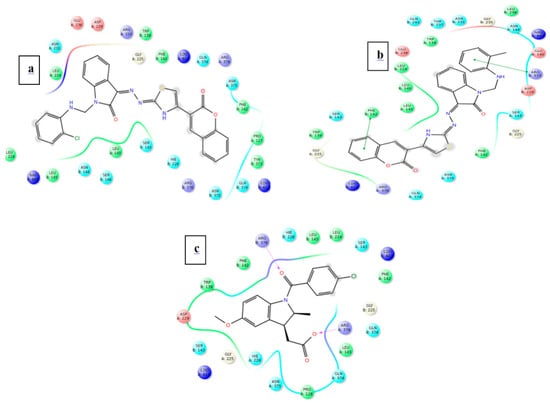
Figure 7.
Ligand interaction of test ligand with the target protein COX-2 from human (a) 4f (b) 4n (c) Indomethacin.
2.2.6.7. ADME Profiling
The suitability of test ligands as drug candidate according to their pharmacokinetic behavior was also assessed using in silico approach. Many drug candidates fail at a later stage due to their poor pharmacokinetic performance. In order to avoid such failure and save time, energy and money in silico ADME profiling is a good choice [44]. The result of in silico ADME profiling is presented in Table 8 that suggests that values of test parameters are within the recommended range (http://glab.cchem.berkeley.edu/glab/schrodinger_old/qikprop/qikprop_user_manual.pdf).

Table 8.
ADMET profiling of different ligands synthesized to be used as drug candidate.
The oral drug absorption is predicted in terms of apparent Caco-2 permeability (QPPlogCaco) that represents the gut-blood barrier. The value above 500 indicates a great absorption while below 25 is considered a poor score [44]. The ligand 4n and 4f haveQPPlogCaco value 619.284 and 479.473 that is very good as compared to indomethacin that has the score of 185.783 only. The Madin-Darby canine kidney (MDCK) cell model is used to investigate the apparent MDCK cell permeability [45]. The score above 500 is considerably good that is obtained in both the test ligand cases. The score for 4n and 4f are 586.303 and 809.359 whereas the standard drug has a value of 251.855. The percent human abortion of both the potential ligand is also comparable to the standard and above 80%. The test ligands are also found to be following Lipinski rule of 5.
2.2.6.8. Statistical Analysis
Data used in the experimental pharmacological section was used as the mean± standard error of the mean (SEM). One way analysis of variance (ANOVA) and Dennett's multiple comparison test techniques was employed to compare between test, control and standard group, utilizing statistical software Graph pad prism version 5.00, California corporation, San Diego, USA. Such results showed significantly different at p < 0.05.
3. Materials and Methods
Melting points were evaluated in open capillary tubes and are uncorrected. 5PC FT-IR spectrometer, Massachusetts, USA, Bruker DRX-300 FT NMR, Bruker, Karlsruhe, Germany) spectrophotometer and Jeol-JMS-D-300 mass spectrometer (70 eV) (Jeol, Tokiyo,Japan) for IR, NMR and mass respectively were used to characterize the compounds.
3.1. Chemistry
3.1.1. Preparation of 2-(2-Oxoindolin-3-Ylidene)Hydrazine Carbothioamide (2)
A combination of isatin (0.01 mole) and thiosemicarbazide (0.01 mole) was placed in 100 mL round bottom flask with 50 mL of methanol as solvent and refluxed for 2 hours and then put onto the ice. The obtained was filtered, dried and recrystallized using methanol.
3.1.2. Preparation of Deep Eutectic Solvent (DES)
A mole ratio (1:2) of choline chloride and urea were chosen to prepare DES as per reported method [28].
3.1.3. Preparation of 3-(2-(4-(2-Oxochroman-3-Yl) Thiazol-2-Yl) Hydrazono) Indolin-2-One Using Deep Eutectic Solvent and Ultrasound (3)
In a specially designed sonicating flask an equimolar(0.01mole) quantity of 3-bromoacetyl coumarin and 2-(2-oxoindolin-3-ylidene) hydrazinecarbothioamide (2) with 8 g of prepared DES was added. A sonicating probe of 26 kHz frequency at 40% amplitude was submerged into the reaction vessel. Completion of the reaction was monitored by taking TLC in regular interval. Upon completion, it was poured onto crushed ice. Upon completion of the reaction, it was extracted by dichloromethane using separating funnel. Organic solvent layer was collected and evaporated to get the desired product. DES was isolated and keeps for future use.
3.1.4. Preparationof1-(Substitutedphenylaminomethyl)-3-(2-(4-(2-Oxochroman-3-yl) Thiazol-2-yl)ydrazono)Indolin-One (4a–n(
A mixture of 3-(2-(4-(2-oxochroman-3-yl)thiazol-2-yl)hydrazono)indolin-2-one (3) (0.01 mole), substituted aromatic amines (0.01 mole) and formaldehyde (0.02 moles) in 30 ml of ethylene glycol was refluxed from 1 hour to 3 hours. The reaction mixture was transferred onto the crushed ice upon completion, as confirmed by TLC. The solid decanted, filtered, washed with water, dried and recrystallized from dioxane.
3.2. Biology
3.2.1. Preparation of 2-(2-Oxoindolin-3-Ylidene)Hydrazine carbothioamide (2)
Compounds produced were assessed for their anti-inflammatory activity using the carrageenan-induced hind paw edema method [45]. The anti-inflammatory activity was carried out using Wistar albino rats of either sex (150–220 g) using Digital Plethysmometer (Model No. 7140, UGO BASILE). The edema was induced by using 1% carrageenan solution. Indomethacin was used as standard drug. The anti-inflammatory activity of the standard drug and tested compounds was determined at a dose of 10 mg/kg body weight. The animals were divided into groups containing 6 animals each and initial paw volume of each rat was noted by NaCl displacement method. One group was kept as control, one as standard and rest groups of the compounds to be tested. To the control group, 1% CMC solution was administered p.o. To the standard group, the standard drug was administered orally. To the test group, tested compounds were administered orally. After 60 minutes of the 1% CMC solution/standard drug/test compound administration, 0.1 ml of 1% (w/v) carrageenan was injected in the plantar region of the hind limb (right) of all the rats in each group including the control group. The paw volume was again measured after the time interval of 2 hours and 4 hours. Using the following formula, inflammation was calculated as percentage inhibition for the test and reference compounds
[Final foot volume of control−Final foot volume of std. / test] × 100 / Final foot volume of control
3.2.2. Analgesic Activity
The analgesic activity of the tested compounds was carried out by acetic acid induced writhing method as given in the literature [9] using Swiss albino mice of either sex (25–35 g). The writh were induced in the albino mice using an intraperitoneal injection of 1% acetic acid solution. The standard drug indomethacin and test compounds were evaluated at a concentration of 10 mg/kg of the body weight. The animals were divided into groups and each group consisted of 6 animals. One group was kept as control, one as standard and other as test groups. To the control group, 0.1% CMC solution was administered p.o; to the standard group standard drug was administered orally, and to the test group test compounds were administered orally. After 60 minutes of the 0.1% CMC solution/standard drug / tested compound administration, 0.1 ml of 1% (v/v) acetic acid solution in distilled water was injected intraperitoneally of all the mice in each group including the control group. The writhing (contraction of the abdomen, turning of trunk and extension of hind limbs) was counted after 5 minutes of acetic acid administration and were counted for a period of 15 minutes. The percentage of analgesic activity was calculated using the following formula.
[(Mean wriths of control−Mean wriths of std./test) / Mean wriths of control] × 100
3.2.3. Acute Ulcerogenic Activity
Acute ulcerogenic activity evaluation of the synthesized compounds was carried out according to the method described [46] using Wistar rats of either sex (180–220 g). The animals were distributed into control, group, and test group. Each group consisted of six rats. All the rats fasted for 24 hours with free access to water. To control group, 1% CMC solution was administered p.o; to the standard group indomethacin at a concentration of 20 mg/kg was administered orally; and to the test, groups tested compounds were administered orally at a concentration of 30 mg/kg. After the dose administration animals were kept for 17 hours. After this, the animals were sacrificed for the appraisal of ulcerogenic assessment. Stomach was taken out from the animal body and washed with flushing water, then with a cotton swab wetted with saline (0.9%) and pinned on wax coated try. Glandular portion of the stomach was cleaned again with saline to closely identify the presence of a type of ulcers or hemorrhage mark using a magnifying glass. The mucosal injury of the stomach was evaluated as per the following system: 0.5 = redness; 1 = spot ulcer; 1.5 = hemorrhage streak; 2 = ulcers < 3; 3 = ulcers > 3 < 5. The value obtained as a result of the mean score of individual treated group - mean score of control is referred to as the severity index of the gastric mucosal damage.
3.2.4. Lipid Peroxidation Study
The method adopted for lipid peroxidation is same as of Ohkawa et al. [47] and recent work of our researchers [9].
3.2.5. Theoretical Details
It is well known that almost all phenolic compounds may inhibit lipid peroxidation process due to their ability to scavenge the chain-carrying lipid peroxyl radicals, LOO•. The lipid, LH, peroxidation process is represented by three main steps initiation, propagation, and terminations. The scavenging of LOO• by the synthesized indolin-2-ones derivatives (In-H) may refer to hydrogen atoms transferred or an electron transfer from the former to the lipid peroxyl radical. The hydrogen atom transfer is represented by the following reaction:
In-H + LOO• → In• + LOOH
The above lipid peroxidation inhibition is governed by bond dissociation enthalpies (BDE) of i-NH groups of the synthesized indolin-2-ones derivatives (In-H). BDE is calculated using the following equation:
where H is the enthalpy that considered as temperature-dependent corrections [zero point energy (ZPE), vibrational, rotational and translational energies at 298K; H (In•, 298K) and H (An-H, 298K) are the enthalpies of In-H derivatives and its corresponding radical obtained after the homolytic bond dissociation of i-NH groups, respectively. H (H•, 298K) is the enthalpy of hydrogen radical. The minimum value of BDE indicates that hemolytic bond dissociation is much easier, which is helpful in lipid peroxidation process
BDE = [H (In•, 298K) + H (H•, 298K)] – H (In-H, 298K),
Previously, we showed the success of the hybrid functional B3B86 in rationalizing the scavenging of free radical by synthesized and natural polyphenols [48,49,50]. Hence, we extended here the use of B3P86 to the In-H synthesized derivatives as lipid peroxyl radical inhibitors. We have already tested, the basic set effect on BDEs of hispidin and isohispidin isomers by using varieties of basic sets. The obtained BDEs showed differences lower than 0.4 kcal/mol for active sites and a slight influence on IP values[48]. Consequently, a double basis set, 6-31+G(d,p), was used in this study. The 3D geometry optimization of In-H derivatives and their corresponding radicals In•were performed at the B3P86/6-6-31+G(d,p) level of theory. The ground state minima were confirmed by vibrational frequency calculations (i.e., theabsence of imaginary frequencies). All DFT chemical calculations have been performed using the mentioned methodology, as implemented in Gaussian 09 package [51].
3.2.6. In-Silico Study
3.2.7. Software
The present in the silico study that includes homology modeling of the target protein, molecular docking, and ADME proofing was carried out using Schrodinger Maestro interface (Maestro, version 10.5, Schrödinger, LLC, New York, NY, USA, 2016) [52,53].
3.2.8. Target Protein Selection and Retrieval
In the present study, COX-2 is selected as the target protein since the therapeutic response of NSAIDs is generated by blocking/inhibiting this enzyme. The 3-D structure of COX-2 was retrieved from Protein Data Bank (PDB, https://www.rcsb.org/). There was two structure of this enzyme, one from mouse and from human origin were obtained, having PDB ID 3NT1 and 5F91 respectively.
3.2.9. Protein (COX-2) Preparation and Validation
The 3-D structure of target proteins that are obtained from PDB was prepared (for further steps) using the tool protein preparation sorcerer (version 4.3), Sage N Research Inc., Milpitas, CA. The protein preparation is a multi-step process that includes the addition of hydrogen atoms, optimization of hydrogen bonds, and elimination of any atomic level clashes. The final step of protein preparation is energy minimization that was performed at the condition 0.3 Å of RMSD and the OPLS_2005 force field [54].
The protein structures prepared in the above step was further validated through a Ramachandran plot based on phi/psi angles evaluation.
3.2.10. Prediction and Evaluation of the Binding Site in COX-2
To locate the position where ligands can bind to the target protein was predicted through Site map application (version 3.8), Schrödinger, New York, USA. The potency of the predicted site is decided on the basis of site score generated by the tool.
The binding site effectiveness is determined by several physical parameters like size, the degree of enclosure/exposure, hydrophobic/hydrophilic character, opportunities of hydrogen bonding, etc.
3.2.11. Ligand Preparation
The derivatives of 1-(substituted phenyl aminomethyl)-3-(2-(4-(2-oxochroman-3-yl) thiazol-2-yl) hydrazono)indolin-2-ones (4a to 4n) that are synthesized chemically in the previous step are used as ligands. The chemical structure of individual ligand was drawn and prepared using LigPrep (version 3.7), Schrödinger, New York, USA. The purpose of ligand preparation is to generate 3-D structure (of each ligand) with minimum energy conformation.
3.2.12. Grid Generation in the Target Protein COX-2
The grid was created nearby the binding site in the respective target proteins that were predicted in the previous step. It determines the exact position and size of the binding site in terms of receptors grids that is required for the docking step. The box size taken is of 20×20×20Å3 and atoms were scaled by van der Waals radii of 1.0 Å having partial atomic charge less than 0.25.
3.2.13. Docking of Ligands and COX-2
The prepared ligands were docked in the COX-2 (target protein) at the respective binding site through Glide (version 7.0), Schrödinger, New York, USA application. The Extra precision (XP) algorithm was employed for the docking operation and output is obtained in the form of docking score. It determines a possible binding pose between the target and the ligand at the binding site along with the information about the most favorable interactions among them [55,56,57].
3.2.14. ADME Profiling
The test ligands i.e. the derivatives of 1-(substitutedphenyl aminomethyl) -3-(2-(4-(2-oxochroman -3-yl) thiazol-2-yl)hydrazono)indolin-2-ones (4a to 4n) were assessed for their pharmacokinetic efficacy through QikProp program (version 4.7), Schrödinger, New York, USA. The tool predicts 51 pharmacokinetic properties but the present study includes a few important parameters that are logP (Octanol/Water), apparent Caco-2 permeability QPP Caco), brain/blood partition coefficient (QPlogBB), apparent MDCK permeability (QppMDCk), (QP%) human oral absorption %, and Lipinski rule of 5 violations (Rule of 5).
4. Conclusions
In conclusion, an improved synthesis of key intermediate through the combined use of deep eutectic solvent and ultrasound is a rational approach to enhance the yield of desired compounds via an economically viable and environmentally acceptable way. Further, all the final compounds (4a-n) have been evaluated as anti-inflammatory and analgesic activities. Selected compounds were further tested for ulcerogenic and lipid peroxidation potential. Only two compounds claimed to be most potent as anti-inflammatory and analgesic molecule with the highest reduction in GI toxicity. Insilico study also supports the utility of these two potent ligands as drug candidate and paves the path for future drug development studies. The active compounds showed similar lipid peroxidation activities, and this mainly due to their closest BDEs and IP values, i.e., the active compounds have the same potency to inhibit lipid radical by a hydrogen atom transfer from the active site of titled compounds to a lipid radical.
Author Contributions
Conceptualization, M.I., M.A., M.B.A. and A.K. (Abida Khan); Synthesis of organic compounds, M.I., M.A. and A.K.; Preparation of DES, Y.R. and M.A.; Biological activities, M.I., M.A. and N.A.; Characterization of organic compounds, M.T.A., M.B.A., and Y.R.; Docking studies, A.V. and A.K. (Awanish Kumar); DFT studies, A.E.H.; Writing original draft and compilation of final draft, M.I., M.A., M.B.A. and A.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
One of the authors (Mohd Imran) is thankful to the Faculty of Pharmacy, JamiaHamdard, New Delhi, India for providing research facility for synthesis and experimental pharmacology. One of author thankful to Chemistry Department, College of science, Prince Sattam Bin Abdulaziz University, Saudi Arabia for DFT results. We are also thankful to the Department of Biotechnology, National Institute of Technology Raipur (CG), India for required docking studies.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds......are available from the authors.
References
- Kumar, N.; Sharma, C.S.; Ranawat, M.S.; Singh, H.P.; Chauhan., L.S.; Dashora, N. Synthesis, analgesic and anti-inflammatory activities of novel mannich bases of benzimidazoles. J. Pharm. Invest. 2015, 45, 65–71. [Google Scholar] [CrossRef]
- Almasirad, A.; Mousavi, Z.; Tajik, M.; Assarzadeh, M.J.; Shafiee, A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. Daru. J. Pharm. Sci. 2014, 22, 1–8. [Google Scholar] [CrossRef]
- Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, S.; Aktay, G.; Özalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic anti- inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Javed, S.A.; Khan, S.A.; Amir, M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: Synthesis and preliminary evaluation of biological properties. Eur. J. Med. Chem. 2008, 43, 2688–2698. [Google Scholar] [CrossRef]
- Marnett, L.J.; Kalgutkar, A.S. Cyclooxygenase 2 inhibitors: Discovery, selectivity and the future Trends. Pharmacol. Sci. 1999, 20, 465–469. [Google Scholar] [CrossRef]
- Marnett, L.J.; Kalgutkar, A.S. Design of selective inhibitors of cyclooxygenase-2 as non ulcerogenic anti-inflammatory agents. Curr. Opin. Chem. Biol. 1998, 2, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Parsit, P.; Reindeau, D. Selective Cyclooxygenase-2 Inhibitors. Annu. Rep. Med. Chem. 1997, 32, 211–220. [Google Scholar]
- Almansa, C.; Alfon, J.; Cavalcanti, F.L. Synthesis and Structure–Activity Relationship of a New Series of COX-2 Selective Inhibitors: 1,5-Diarylimidazoles. J. Med. Chem. 2003, 46, 3463–3475. [Google Scholar] [CrossRef]
- Alafeefy, A.M.; Bakht, M.A.; Ganaie, M.A.; Ansarie, M.N.; NEl-Sayed, N.N.; Awaad, A.S. Synthesis, analgesic, anti-inflammatory and anti-ulcerogenic activities of certain novel Schiff’s bases as fenamateisosteres. Bioorg. Med. Chem. Let. 2015, 25, 179–183. [Google Scholar] [CrossRef]
- Kujubu, D.A.; Fletcher, B.S.; Varnuzu, B.; Lim, R.W.; Herschmann, H.R. TIS 10, a phorbol ester tumor promotor-inducible mRNA from Swiss 3T3 cells encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Bio. Chem. 1991, 266, 12866–12872. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Akarasereenont, P.; Thiemermann, C.; Flower, R.J.; Vane, J.R. Selectivity of non-steroidalanti- inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Nat. Acad. Sci. USA 1993, 90, 11693–11697. [Google Scholar] [CrossRef]
- Laine, L. Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest. Endosc. Clin. N. Am. 1996, 6, 489–504. [Google Scholar] [CrossRef]
- Fries, J.F.; Williams, C.A.; Bloch, D.A. The relative toxicity of nonsteroidal anti-inflammatory drugs. Arthr. Rhem. 1991, 34, 1353–1360. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, D.; Singh, S.; Sharma, V.; Gaba, P. Synthesis and pharmacological evaluation of novel 5-substituted-1-(phenylsulfonyl)-2-methylbenzimidazole derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 2245–2249. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Sujatha, K.; Perumal, P.T.; Muralidharan, D.; Rajendran, M. Synthesis, analgesic and anti-inflammatory activities of bi(indolyl) methanes. Ind. J. Chem. 2009, 48B, 267–272. [Google Scholar]
- Nirmal, R.; Prakash, C.R.; Meenakshi, K.; Shanmugapandiyan, P. Synthesis and Pharmacological Evaluation of Novel Schiff Base Analogues of 3-(4-amino) Phenylimino) 5-fluoroindolin-2-one. J. Young. Pharm. 2010, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.S.; More, H.N.; Bhosale, A.V. Synthesis, Characterization and Evaluation of Analgesic and Anti-inflammatory Activities of Some Novel Indoles. Trop. J. Pharm. Res. 2011, 10, 463–473. [Google Scholar] [CrossRef]
- Dubey, P.K.; Kumar, T.V. Synthesis of [2-(3-oxo-3,4-dihydro-2H-benzo[1,4] oxazin-6-carbonyl)-1-1H-indol-3-yl]acetic acids as potential COX-2 inhibitors. Ind. J. Chem. 2006, 45B, 2128–2132. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Pandey, P. A facile synthesis of N-phenyl-6-hydroxy-3-bromo-4-arylazo under phase transfer catalytic conditions and studies on their antimicrobial activities. Ind. J. Chem. 2006, 45B, 2077–2082. [Google Scholar] [CrossRef]
- Mladenova, R.; Ignatova, M.; Manolova, N.; Petrova, T.; Rashkov, I. Preparation, characterization and biological activity of Schiff base compounds derived from 8-hydroxyquinoline-2-carboxaldehyde and Jeffamines ED. Eur. Polym. J. 2002, 38, 989–999. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D.; Akram, M.; Kaur, H. Synthesis and evaluation of some newer indole derivatives as anticonvulsant agents. Int. J. Pharm. Bio. Arch. 2011, 2, 744–750. [Google Scholar]
- Jayashree, B.S.; Nigam, S.; Pai, A.; Chowdary, P.V.R. Overview on the recently developed coumarinylheterocycles as useful therapeutic agents. Arabian. J. Chem. 2014, 7, 885–899. [Google Scholar] [CrossRef]
- Xin-Mei, P.; Guri, L.V.D.; Cheng, H.Z. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. 2013, 19, 3884–3930. [Google Scholar]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Bio. Med. Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Sobhi, M.G.; Khaled, D.K. A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity. Molecules 2012, 17, 9335–9347. [Google Scholar]
- Thoraya, A.F.; Maghda, A.A.; Ghada, S.M.; Zeinab, A.M. New and efficient approach for synhesis of novel bioactive [1,3,4] thiadiazoles incorporated with 1,3-thiazole moiety. Eur. J. Med. Chem. 2015, 97, 320–333. [Google Scholar]
- Singh, B.S.; Lobo, H.R.; Pinjari, D.V.; Jaraq, K.J.; Pandit, A.B.; Shankarling, G.S. Ultrasound and deep eutectic solvent (DES): A novel blend of techniques for rapid and energy efficient synthesis of oxazoles. Ultrason. Sonochem. 2013, 1, 287–293. [Google Scholar] [CrossRef]
- Bakht, M.A.; Ansari, M.J.; Riaydi, Y.; Ajmal, N.; Ahsan, M.J.; Shahar Yar, M. Physicochemical characterization of benzalkonium chloride and urea based deep eutectic solvent (DES): A novel catalyst for the efficient synthesis of isoxazolines under ultrasonic irradiation. Mol. Liquid. 2016, 224, 1249–1255. [Google Scholar] [CrossRef]
- Wang, S.Y.; Su, X.M. A meldrum’s acid catalyzed synthesis of Bis (Indolyl) methanes in water under ultrasonic condition. Chin. J. Chem. 2008, 26, 22–24. [Google Scholar] [CrossRef]
- Zang, H.; Zhang, Y.; Cheng, B.W. An efficient ultrasound- promoted method for the one-pot synthesis of 7,10,11,12-tetrahydrobenzo [C] acridin-8 (9) –one derivatives. Ultrason. Sonochem. 2010, 17, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, S.A. Antiinflammatory activity of some new 3-(2H-1-benzopyran-2-one-3-yl)-5-substituted aryl isoxazolines. Asian J. Chem. 2004, 16, 543–545. [Google Scholar]
- El-Feky, H.A.S.; Imran, M.; Osman, A.N. Guanidine-annellatedheterocycles: Design, synthesis and biological evaluation of novel 2-aminopyridoimidazotriazines, pyridoimidazopyrimidines, pyridoimidazotriazoles and pyridoimidazoles. BAO. J. Pharm. Sci. 2015, 1, 1–11. [Google Scholar]
- Gupta, S.K.; Imran, M.; Rehman, Z.U.; Khan, S.A. Synthesis and biological evaluation of some 2-substituted benzimidazoles. Asian J. Chem. 2005, 17, 2741–2747. [Google Scholar]
- Alam, O.; Gupta, S.K.; Imran, M.; Khan, S.A. Synthesis and biological activity of some 2,5-disubstituted 1,3,4-oxadiazoles. Asian J. Chem. 2005, 17, 1281–1286. [Google Scholar]
- Abbas, M.A. Synthesis of Sulfonyl 2-(prop-2-yn-1-yl thio)benzo [d] thiazole derivatives. Al-Nahrain. J. Sci. 2015, 18, 74–78. [Google Scholar]
- Mishra, D.; Fatima, A.; Rout, C.; Sing, R. An efficient one-pot synthesis of 2-aminothiazole derivatives. Der. Chem. Sin. 2015, 6, 14–18. [Google Scholar]
- Abdelriheem, N.A.; Mohamed, A.M.M.; Abdelhamid, A.O. Synthesis of some new 1,3,4-Thiadiazole, Thiazole and pyridine derivatives containing 1,2,3-Triazole moiety. Molecules 2017, 22, 1–15. [Google Scholar] [CrossRef]
- Bakht, M.A. Synthesis of some oxadiazole derivatives using conventional solvent and deep eutectic solvent (DES) under ultrasonic irradiation: A comparative study. Adv. Biores. 2016, 7, 64–68. [Google Scholar]
- Bakht, M.A. Ultrasound mediated synthesis of some pyrazoline derivatives using biocompatible deep eutectic solvent (DES). Der. Pharm. Chem. 2015, 7, 274–278. [Google Scholar]
- Pohle, T.; Brzozowski, T.; Becker, J.C.; VanderVoort, I.R.; Markmann, A.; Konturek, S.J.; Moniczewski, A.; Domschke, W.; Konturek, J.W. Role of reactive oxygen metabolites in aspirin-induced gastric damage in humans: Gastroprotection by vitamin C. Aliment. Pharmacol. Ther. 2001, 15, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Duggan, K.C.; Walters, M.J.; Musee, J.; Harp, J.M.; Kiefer, J.R.; Oates, J.A.; Marnett, L.J. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J. Biol. Chem. 2010, 285, 34950–34959. [Google Scholar] [CrossRef]
- Lucido, M.J.; Orlando, B.J.; Vecchio, A.J.; Malkowski, M.G. Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight in to the Formation of Products with Reversed Stereochemistry. Biochem. 2016, 55, 1226–1238. [Google Scholar] [CrossRef]
- Hou, T.J.; Zhang, W.; Xia, K.; Qiao, X.B.; Xu, X.J. ADME evaluation in drug discovery.5. Correlation of caco-2 permeation with simple molecular properties. J. Chem. Inf. Comput. Sci. 2004, 44, 1585–1600. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar] [PubMed]
- Cioli, V.; Putzolu, S.; Rossi, V.; ScorzaBarcellona, P.; Corradino, C. The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol. Appl. Pharmacol. 1979, 50, 283–289. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.; Calliste, C.; Kosinova, P.; Di Meo, F.; Duroux, J.; Champavier, Y.; Marakchi, K.; Trouillas, P. Free radical scavenging properties of guaiacol oligomers: A combined experimental and quantum study of the guaiacyl-moiety role. J. Phys. Chem. A. 2009, 113, 13881–13891. [Google Scholar] [CrossRef]
- Anouar, E.H. A Quantum Chemical and Statistical Study of Phenolic Schiff Bases with Antioxidant Activity against DPPH Free Radical. Antioxidants 2014, 3, 309–322. [Google Scholar] [CrossRef]
- Anouar, E.H.; Shah, S.A.A.; Hassan, B.; Moussaoui, N.E.; Ahmad, R.; Zulkefeli, M.; Weber, J.F.F. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules 2014, 19, 3489–3507. [Google Scholar] [CrossRef]
- Trucks., G.W.; Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 2002, 3220, 597–608. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all –atom protein loop prediction. Proteins: Struct. Funct. Bioinf. 2004, 367, 351–367. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Sherman, W. Protein and ligand preparation: Parameters protocols and influence on virtual screening enrichments. J. Comput. Aided. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repask, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. A new approach for rapid, accurate docking and scoring 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporation a Model of Hydrophobic Enclosure for Protein –Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and scoring.2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).