Ionic Liquid Mediated Ugi/SN2 Cyclization: Synthesis of 1,2,3-Triazole Containing Novel 2,5-Diketopiperazines †
Abstract
1. Introduction
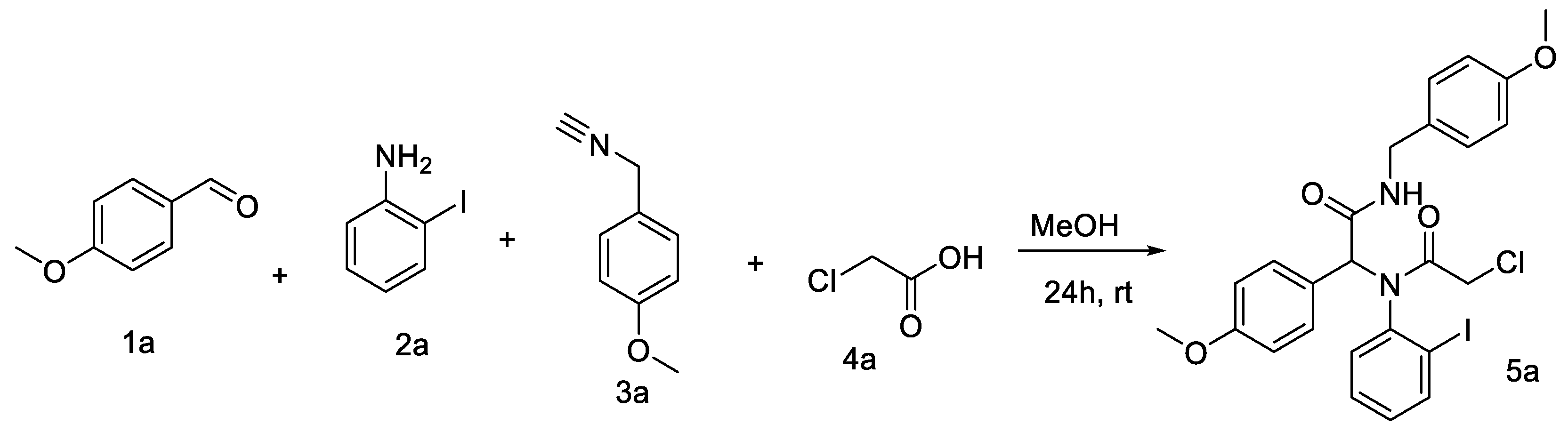
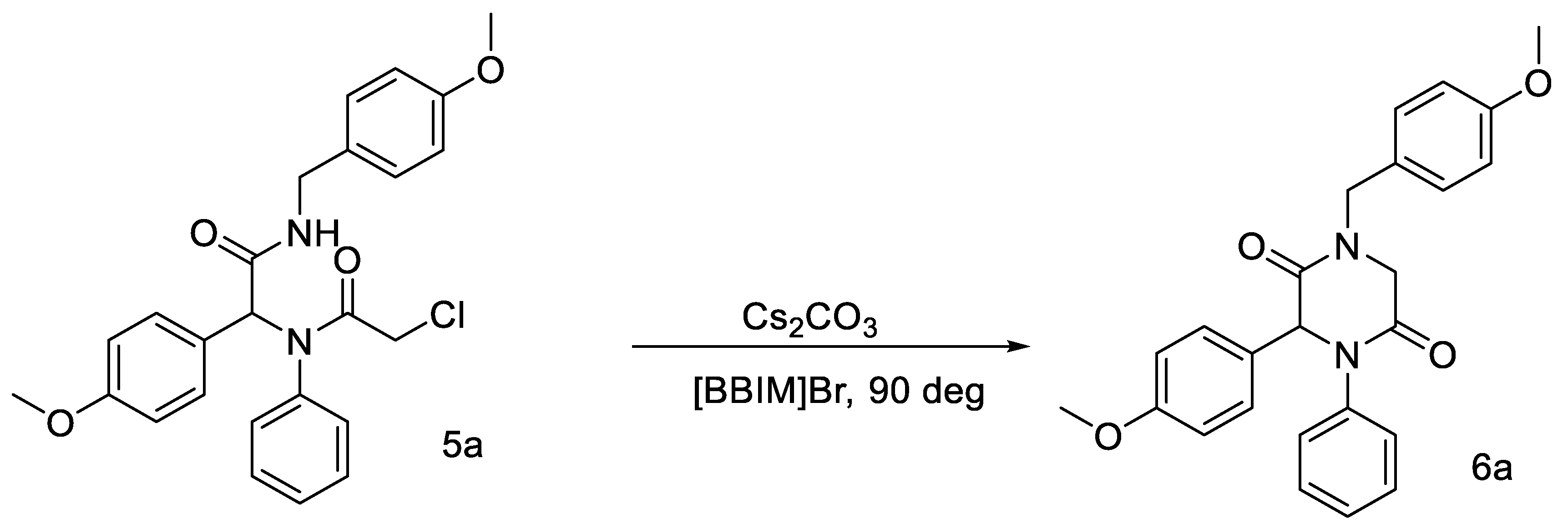
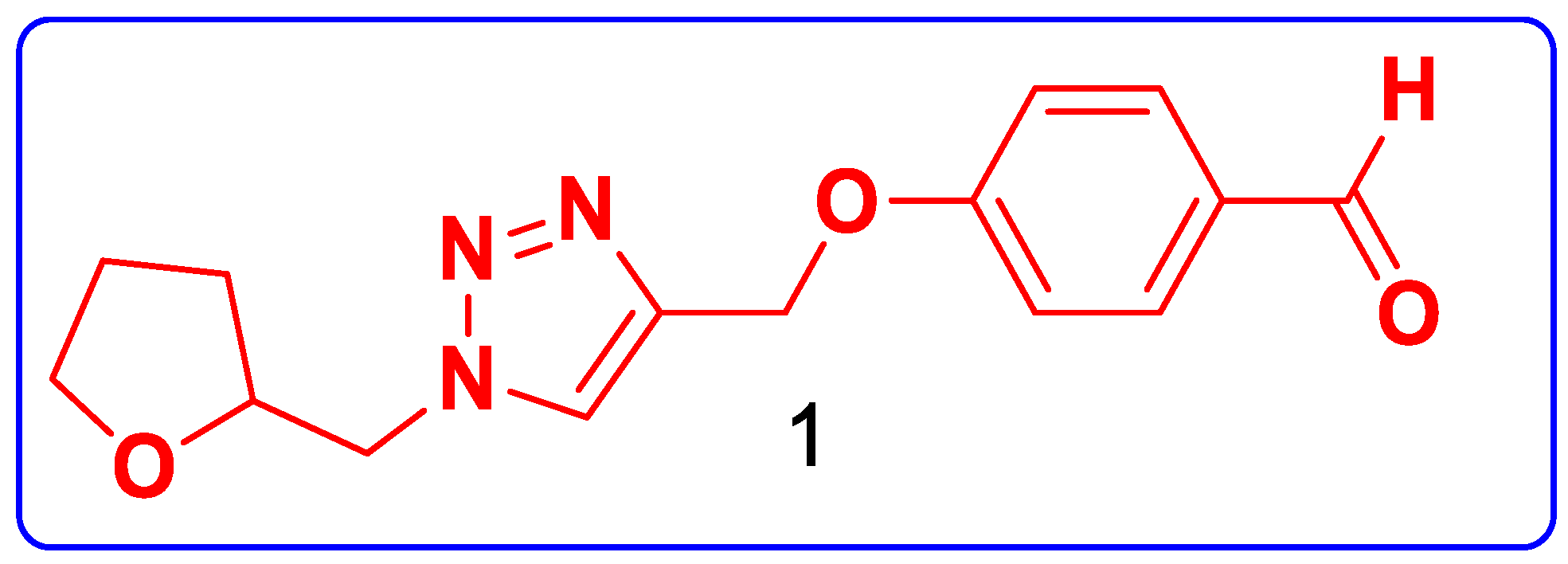
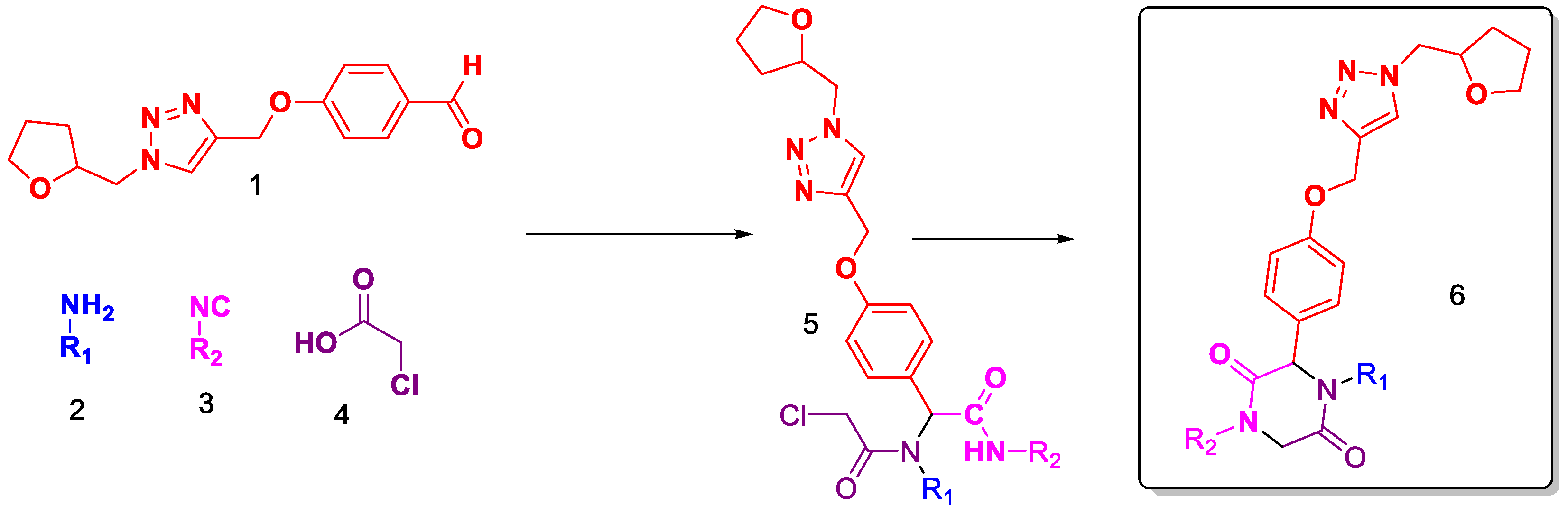
2. Results and Discussion


3. General Experimental Procedure
3.1. Synthesis of Ugi Adduct 5
3.2. Synthesis of 2,5-Diketopiperazine Derivative 6
4. Conclusions
Supplementary Materials
References
- Dömling, A.; Ugi, I. Multicomponent reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Hulme, C.; Gore, V. Multi-component reactions: Emerging chemistry in drug discovery from Xylocain to Crixivan. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bienymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, G.; Ruijter, E.; Orru, R.V.A. Efficiency, diversity, and complexity with multicomponent reactions. Synlett 2013, 24, 666–685. [Google Scholar]
- Appkkuttan, P.; Dehaen, W.; Fokin, V.V.; van der Eycken, E. A microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper (I)-catalyzed three-component reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef] [PubMed]
- García-Vanegas, J.J.; Ramírez-Villalva, A.; Fuentes-Benites, A.; MartÍnez-Otero, D.; González-Rivas, N.; Cuevas-Yañez, E. Synthesis and in-vitro biological evaluation of 1,1-diaryl-2-(1,2,3)triazol-1-yl-ethanol derivatives as antifungal compounds flutriafol analogues. J. Chem. Sci. 2019, 131, 27. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Pingaew, R.; Anuwongcharoen, N.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Discovery of novel 1,2,3-triazole derivatives as anticancer agents using QSAR and in silico structural modification. Springerplus 2015, 4, 571. [Google Scholar] [CrossRef] [PubMed]
- Lakkakula, R.; Roy, A.; Mukkanti, K.; Sridhar, G. Synthesis and Anticancer Activity of 1,2,3-Triazole Fused N-Arylpyrazole Derivatives. Russ. J. Gen. Chem. 2019, 89, 831–835. [Google Scholar] [CrossRef]
- López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; Masłyk, M.; Estévez-Braun, A. Synthesis and Antimicrobial Activity of 4-Substituted 1,2,3-Triazole-Coumarin Derivatives. Molecules 2018, 23, 199. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Choi, J.; Choi, S.-J.; Baek, K.-H. Cyclodipeptides: An Overview of Their Biosynthesis and Biological Activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Laali, K.K. Chapter Five-Ionic liquid-mediated synthesis and functionalization of heterocyclic compounds. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 128, pp. 333–431. [Google Scholar]
- Lin, Q.; Blackwell, H.E. Rapid synthesis of diketopiperazine macroarrays via Ugi four-component reactions on planar solid supports. Chem. Commun. 2006, 27, 2884–2886. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Keum, G.; Kang, S.B.; Han, S.-Y.; Kim, Y. An efficient synthesis of 2,5-diketopiperazine derivatives by the Ugi four-center three-component reaction. Mol. Divers. 2003, 6, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Vroemans, R.; Bamba, F.; Winters, J.; Thomas, J.; Jacobs, J.; Van Meervelt, L.; John, J.; Dehaen, W. Beilstein. J. Org. Chem. 2018, 14, 626–633. [Google Scholar] [CrossRef]
- Garrido, M.; Corredor, M.; Orzáez, M.; Alfonso, I.; Messeguer, A. Regioselective Synthesis of a Family of β-Lactams Bearing a Triazole Moiety as Potential Apoptosis Inhibitors. ChemistryOpen 2016, 5, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Stucchi, M.; Cairati, S.; Cetin-Atalay, R.; Christodoulou, M.S.; Grazioso, G.; Pescitelli, G.; Silvani, A.; Yildirim, D.C.; Lesma, G. Application of the Ugi reaction with multiple amino acid-derived components: Synthesis and conformational evaluation of piperazine-based minimalist peptidomimetics. Org. Biomol. Chem. 2015, 13, 4993–5005. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnamatla, M.V.B.; García-Eleno, M.A.; Yáñez, E.C. Ionic Liquid Mediated Ugi/SN2 Cyclization: Synthesis of 1,2,3-Triazole Containing Novel 2,5-Diketopiperazines. Proceedings 2019, 41, 75. https://doi.org/10.3390/ecsoc-23-06657
Unnamatla MVB, García-Eleno MA, Yáñez EC. Ionic Liquid Mediated Ugi/SN2 Cyclization: Synthesis of 1,2,3-Triazole Containing Novel 2,5-Diketopiperazines. Proceedings. 2019; 41(1):75. https://doi.org/10.3390/ecsoc-23-06657
Chicago/Turabian StyleUnnamatla, M. V. Basavanag, Marco A. García-Eleno, and Erick Cuevas Yáñez. 2019. "Ionic Liquid Mediated Ugi/SN2 Cyclization: Synthesis of 1,2,3-Triazole Containing Novel 2,5-Diketopiperazines" Proceedings 41, no. 1: 75. https://doi.org/10.3390/ecsoc-23-06657
APA StyleUnnamatla, M. V. B., García-Eleno, M. A., & Yáñez, E. C. (2019). Ionic Liquid Mediated Ugi/SN2 Cyclization: Synthesis of 1,2,3-Triazole Containing Novel 2,5-Diketopiperazines. Proceedings, 41(1), 75. https://doi.org/10.3390/ecsoc-23-06657





