Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones Via Ugi-Azide Reaction Catalyzed by ZnO Nanoparticles †
Abstract
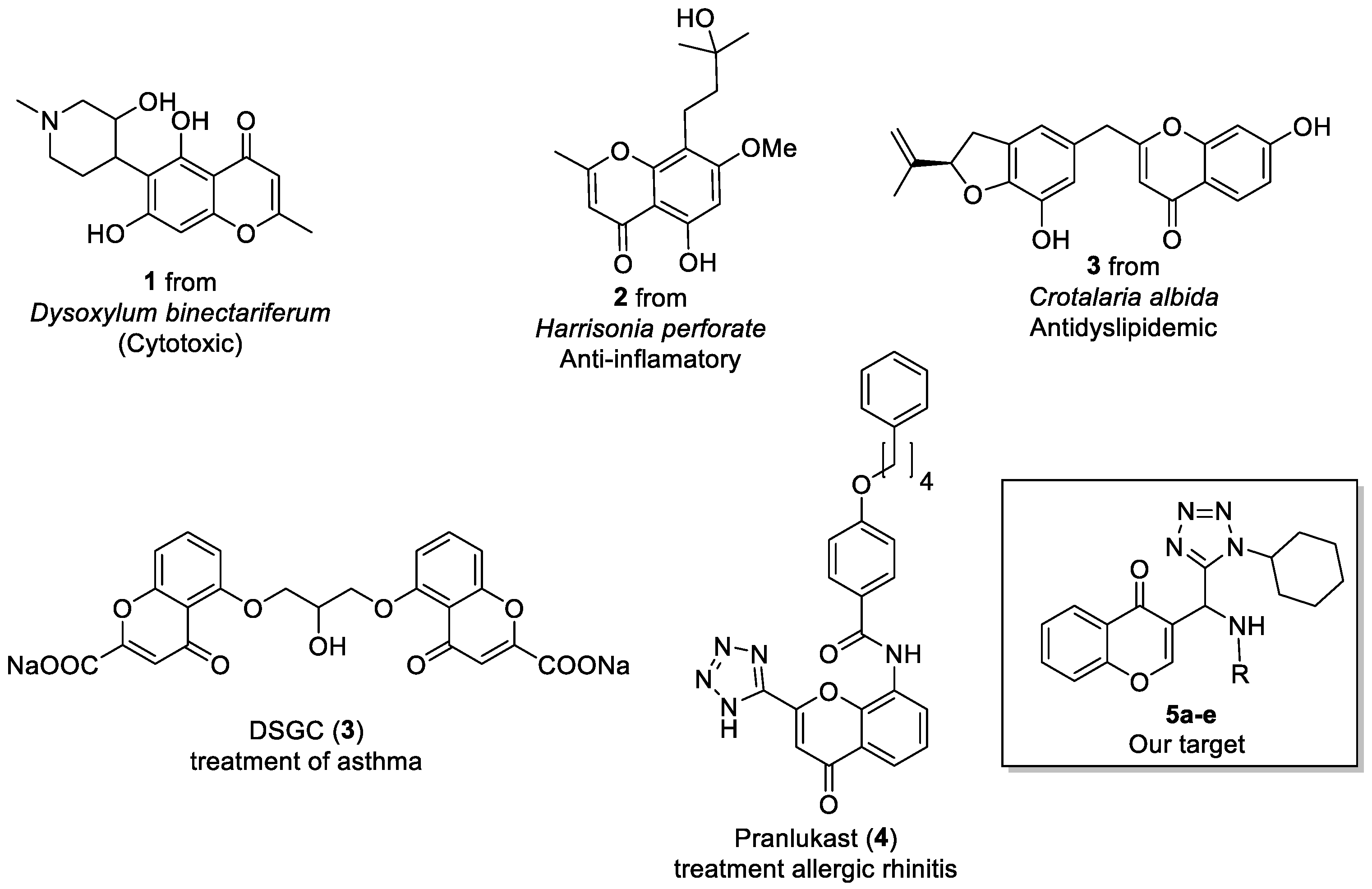
:1. Introduction
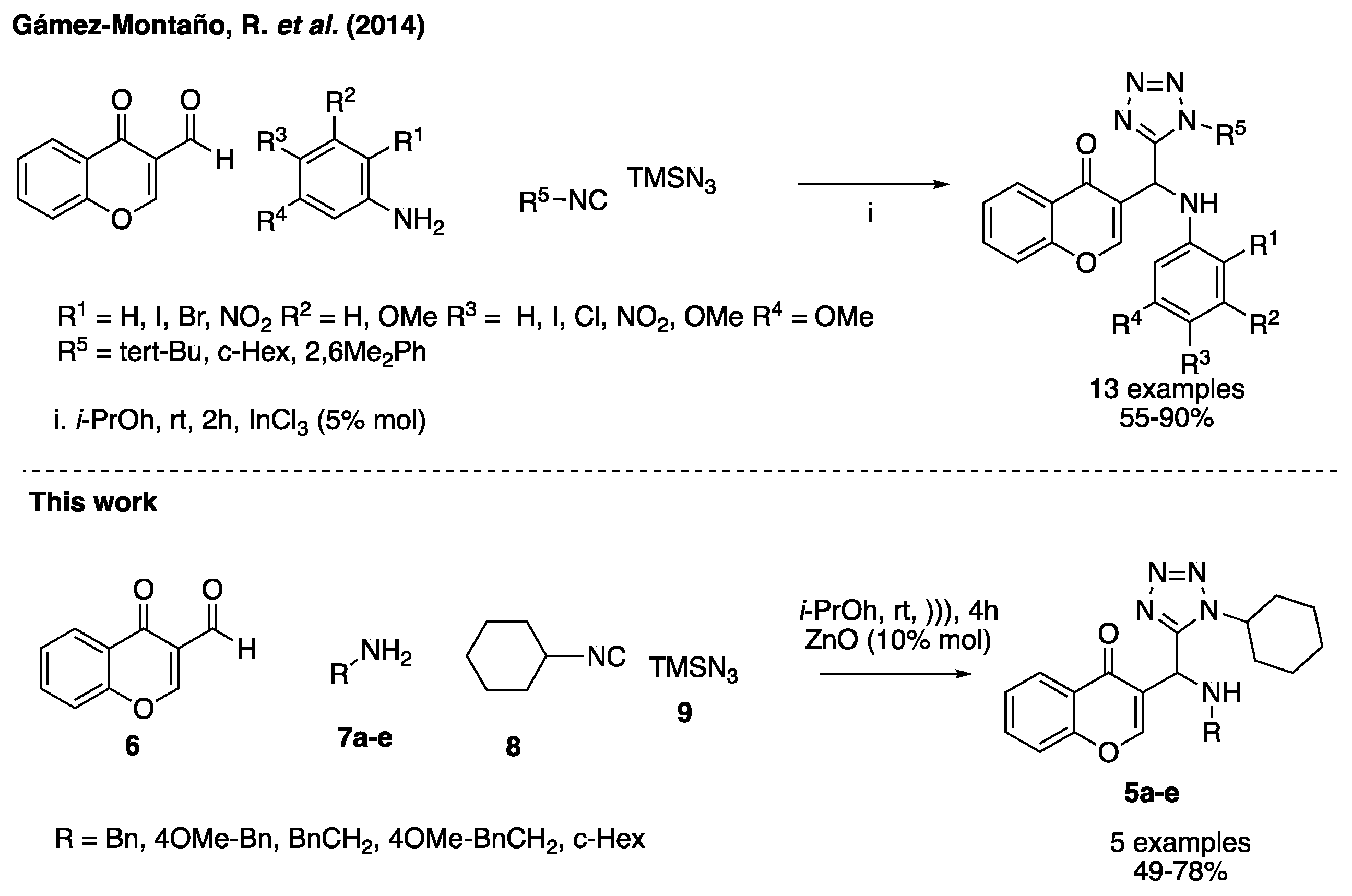
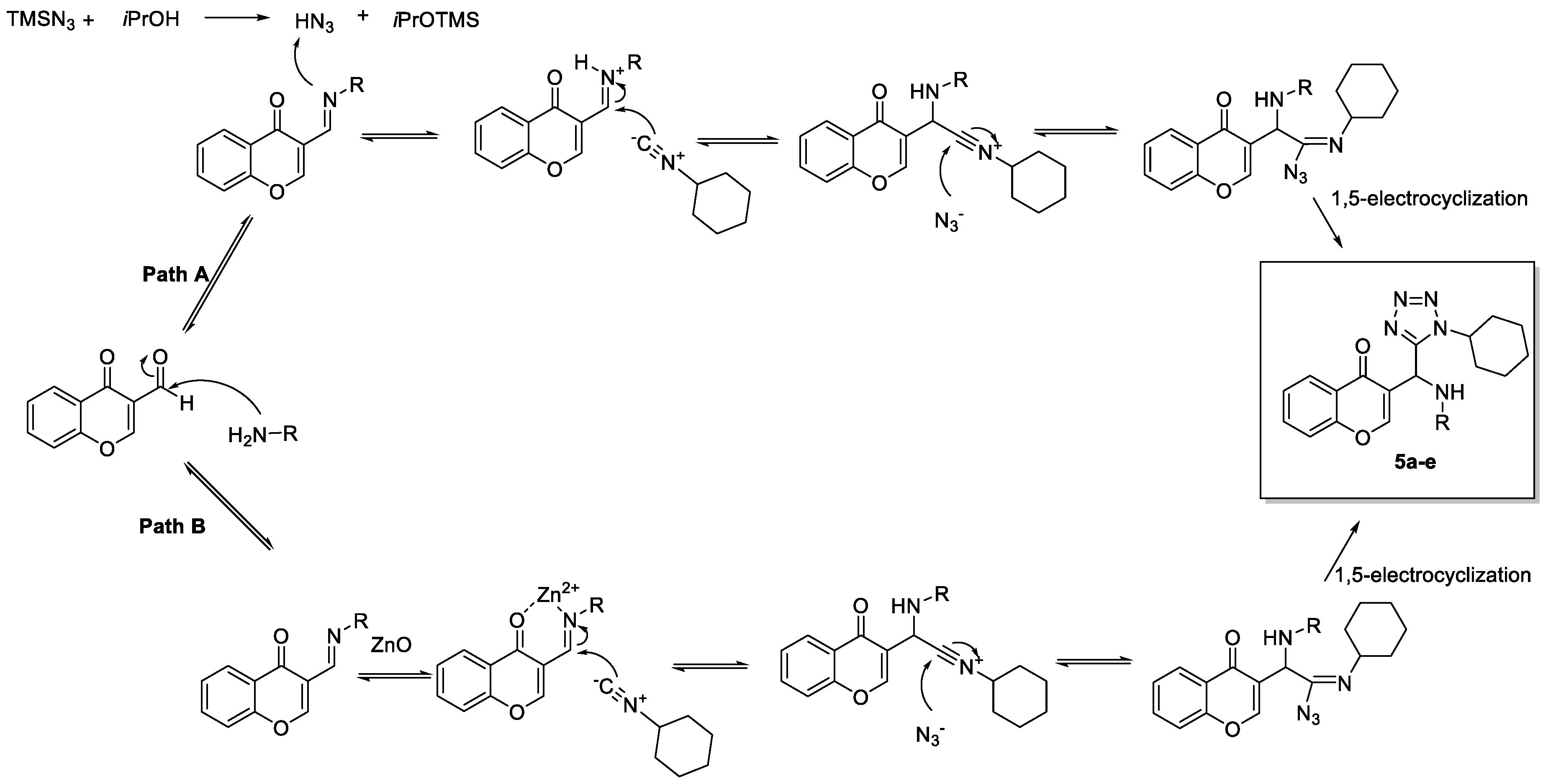
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
3.2. Synthesis and Characterization of the 3-Tetrazolylmethyl-4H-Chromen-4-Ones 5a–e
3-((benzylamino)(1-cyclohexyl-1H-tetrazol-5-yl)methyl)-4H-chromen-4-one (5a)
3-((1-cyclohexyl-1H-tetrazol-5-yl)((4-methoxybenzyl)amino)methyl)-4H-chromen-4-one (5b)
3-((1-cyclohexyl-1H-tetrazol-5-yl)(phenethylamino)methyl)-4H-chromen-4-one (5c)
3-((1-cyclohexyl-1H-tetrazol-5-yl)((4-methoxyphenethyl)amino)methyl)-4H-chromen-4-one (5d)
3-((1-cyclohexyl-1H-tetrazol-5-yl)(cyclohexylamino)methyl)-4H-chromen-4-one (5e)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Romano, R.; Mobilia, M.A.; Izzo, A.A.; Santini, A. Human health-related properties of chromones: An overview. Nat. Prod. Res. 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Jiang, Y.; Guo, F.; Chen, L.; Xu, L.; Zhang, W.; Liu, B. The antitumor activity of naturally occurring chromones: A review. Fitoterapia 2019, 135, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Herr, R.J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorganic Med. Chem. 2002, 10, 3379–3393. [Google Scholar] [CrossRef] [PubMed]
- Ostrovskii, V.A.; Trifonov, R.E.; Popova, E.A. Medicinal chemistry of tetrazoles. Russ. Chem. Bull. 2012, 61, 768–780. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound assisted green synthesis of bound type bis-heterocyclic carbazolyl imidazo[1,2-a]pyridines via Groebke-Blackburn-Bienayme reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]
- Claudio-Catalán, M.A.; Pharande, S.G.; Quezada-Soto, A.; Kishore, K.G.; Rentería-Gómez, A.; Padilla-Vaca, F.; Gámez-Montaño, R. Solvent- and Catalyst-Free One-Pot Green Bound-Type Fused Bis-Heterocycles Synthesis via Groebke–Blackburn–Bienaymé Reaction/SNAr/Ring-Chain Azido-Tautomerization Strategy. ACS Omega 2018, 3, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Kaveti, B.; Ramírez-López, S.C.; Gámez Montaño, R. Ultrasound-assisted green one-pot synthesis of linked bis-heterocycle peptidomimetics via IMCR/post-transformation/tandem strategy. Tetrahedron Lett. 2018, 59, 4355–4358. [Google Scholar] [CrossRef]
- Shaabani, A.; Hooshmand, S.E. Diversity-oriented catalyst-free synthesis of pseudopeptides containing rhodanine scaffolds via a one-pot sequential isocyanide-based six-component reactions in water using ultrasound irradiation. Ultrason. Sonochem. 2018, 40, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pharande, S.G.; Corrales Escobosa, A.R.; Gámez-Montaño, R. Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction. Green Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]
- Chandgude, A.L.; Narducci, D.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Dömling, A. Diastereoselective one pot five-component reaction toward 4-(tetrazole)-1,3-oxazinanes. RSC Adv. 2017, 7, 49995–49998. [Google Scholar] [CrossRef] [PubMed]
- Unnamatla, M.V.B.; Islas-Jácome, A.; Quezada-Soto, A.; Ramírez-López, S.C.; Flores-Álamo, M.; Gámez-Montaño, R. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo[1,2-a]pyridin Tetrazolo[1,5-a]quinolines. J. Org. Chem. 2016, 81, 10576–10583. [Google Scholar] [CrossRef] [PubMed]
- Chandgude, A.L.; Dömling, A. An efficient Passerini tetrazole reaction (PT-3CR). Green Chem. 2016, 18, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Keshipour, S.; Shaabani, A.; Shojaei, S. Nosrati, H.; Ng S.W. A novel one-pot isocyanide-based three-component reaction: synthesis of highly functionalized imidazo-chromen-4-ones. J. Iran Chem. Soc. 2015, 1655–1663. [Google Scholar] [CrossRef]
- Cano, P.A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Synthesis of 3-tetrazolylmethyl-4H-chromen-4-ones via Ugi-azide and biological evaluation against Entamoeba histolytica, Giardia lamblia and Trichomona vaginalis. Bioorganic Med. Chem. 2014, 22, 1370–1376. [Google Scholar] [CrossRef] [PubMed]

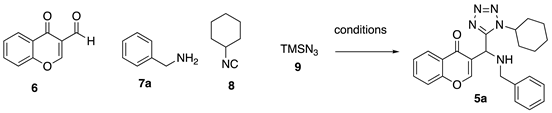 | |||||
|---|---|---|---|---|---|
| Entry a | Solvent b | Aditive c | T (°C) | Time (h) | Yield (%) d |
| 1 | methanol | — | rt | 12 | 37 |
| 2 | ethanol | — | rt | 12 | 33 |
| 3 | isopropanol | — | rt | 12 | 46 |
| 4 | isopropanol | ZnO | rt USI | 1 | 45 |
| 5 | isopropanol | ZnO | rt USI | 4 | 78 |
| 6 | isopropanol | ZnO | 60 USI | 4 | 76 |
| 7 | isopropanol | NH4Cl | rt USI | 6 | 39 |
| 8 | isopropanol | TsOH | rt USI | 6 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentería-Gómez, M.A.; Gámez-Montaño, R. Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones Via Ugi-Azide Reaction Catalyzed by ZnO Nanoparticles. Proceedings 2019, 41, 69. https://doi.org/10.3390/ecsoc-23-06517
Rentería-Gómez MA, Gámez-Montaño R. Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones Via Ugi-Azide Reaction Catalyzed by ZnO Nanoparticles. Proceedings. 2019; 41(1):69. https://doi.org/10.3390/ecsoc-23-06517
Chicago/Turabian StyleRentería-Gómez, Manuel A., and Rocío Gámez-Montaño. 2019. "Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones Via Ugi-Azide Reaction Catalyzed by ZnO Nanoparticles" Proceedings 41, no. 1: 69. https://doi.org/10.3390/ecsoc-23-06517
APA StyleRentería-Gómez, M. A., & Gámez-Montaño, R. (2019). Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones Via Ugi-Azide Reaction Catalyzed by ZnO Nanoparticles. Proceedings, 41(1), 69. https://doi.org/10.3390/ecsoc-23-06517





