Synthesis of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines via Ultrasound-Assisted One-Pot Ugi-azide/Pictet–Spengler Process †
Abstract
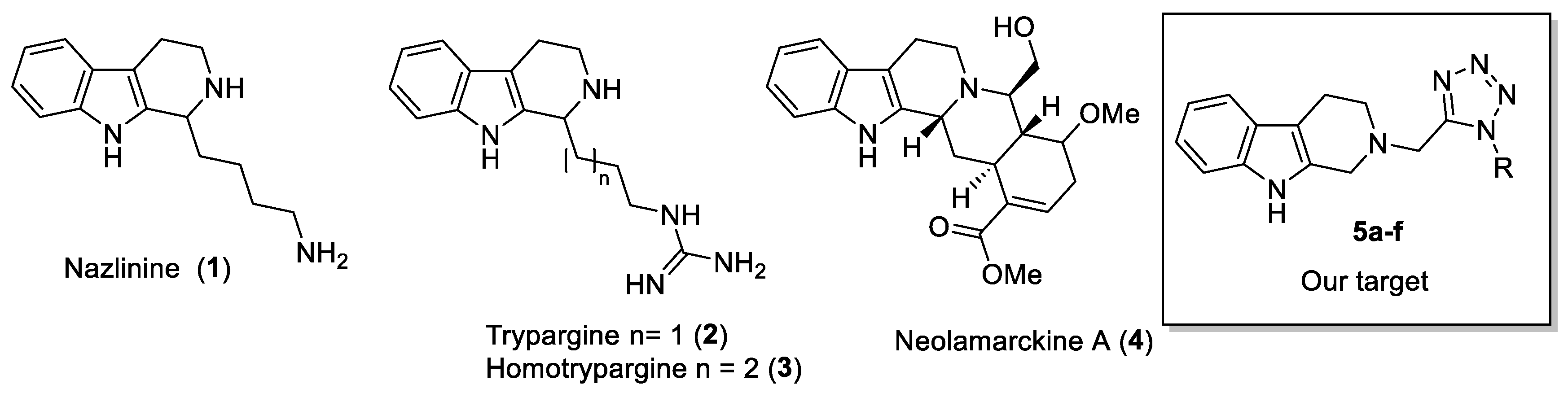
1. Introduction
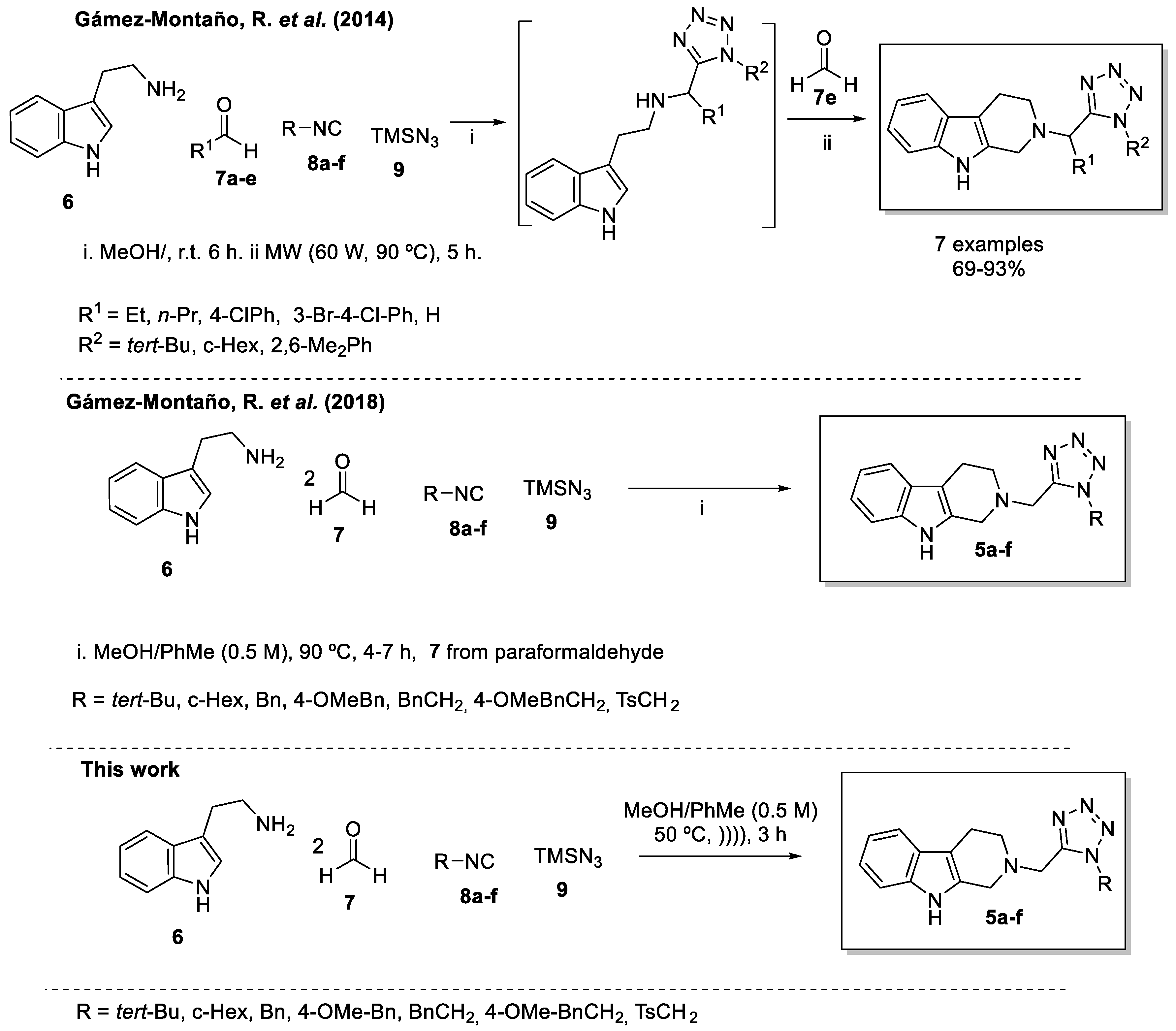
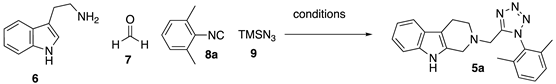
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
3.2. Synthesis and Characterization of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines 5a–f
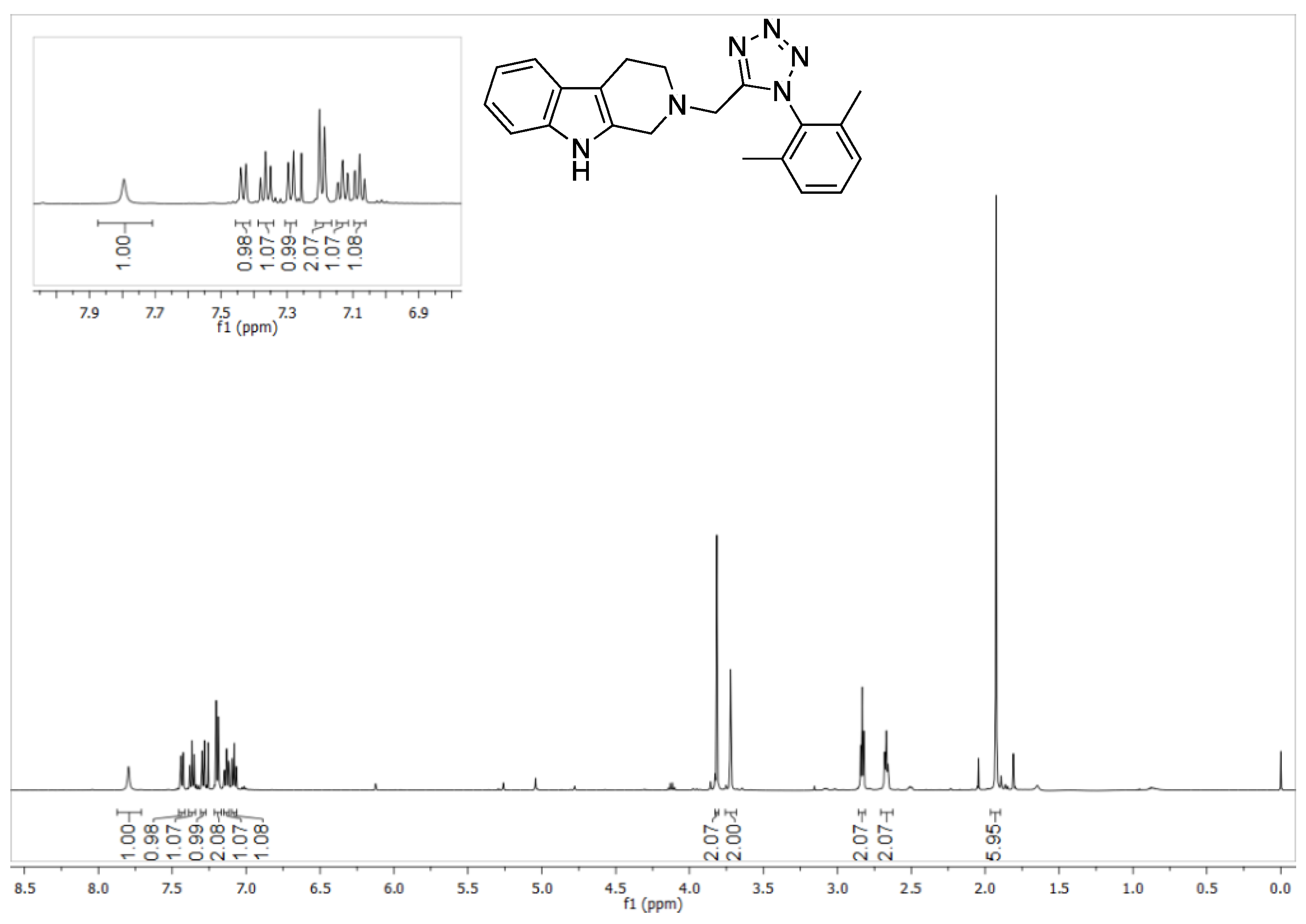
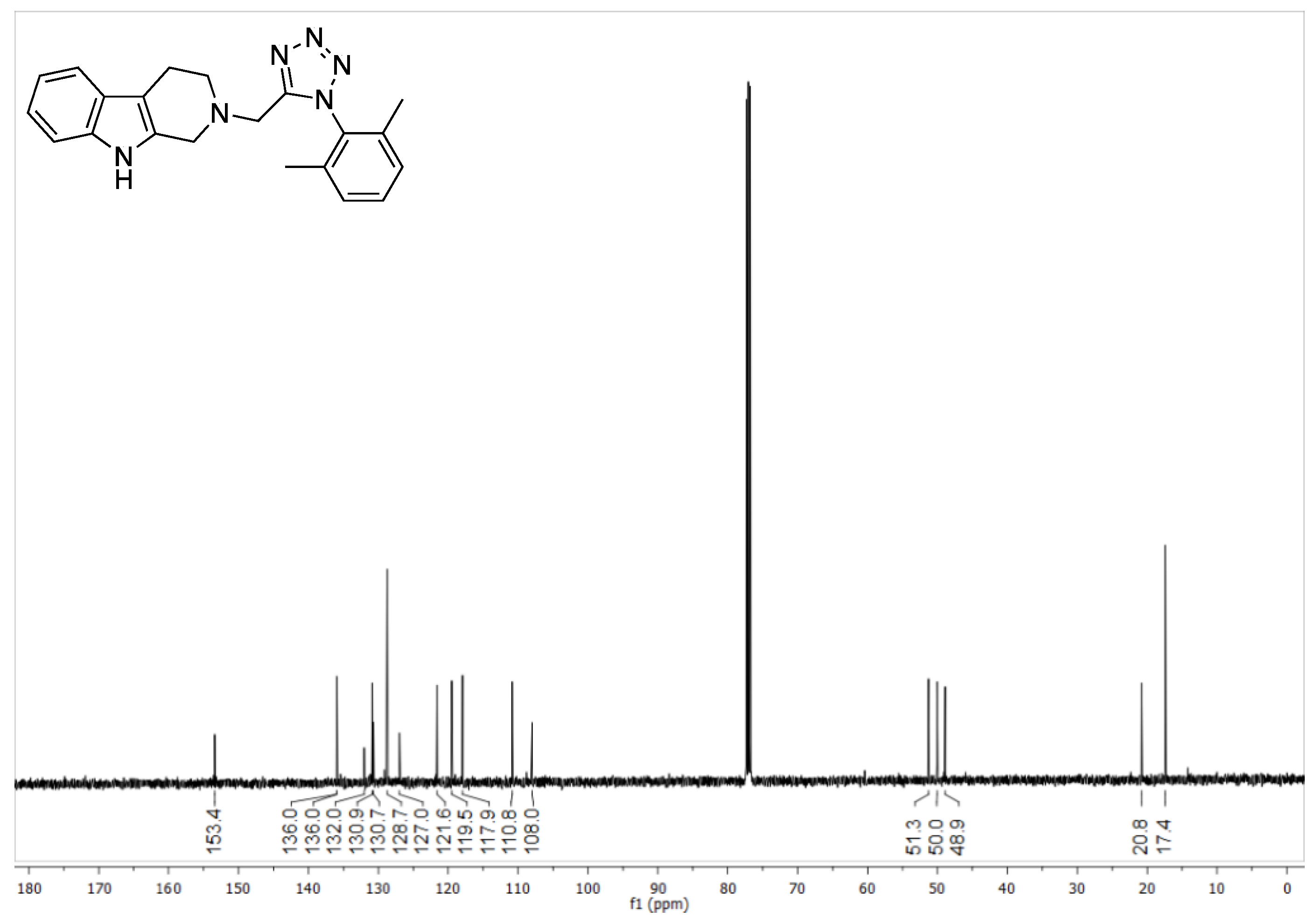
2-((1-(2,6-dimethylphenyl)-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido [3,4-b] indole (5a)
2-((1-cyclohexyl-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (5b)
2-((1-(tert-butyl)-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (5c)
2-((1-benzyl-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (5d)
2-((1-(4-methoxybenzyl)-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (5e)
2-((1-(tosylmethyl)-1H-tetrazol-5-yl)methyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (5f)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shmatova, O.I.; Khrustalev, V.N.; Nenajdenko, V.G. From Cyclic CF3-ketimines to a Family of Trifluoromethylated Nazlinine and Trypargine Analogues. Org. Lett. 2016, 18, 4494–4497. [Google Scholar] [CrossRef] [PubMed]
- Herr, R.J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. [Google Scholar] [CrossRef] [PubMed]
- Ostrovskii, V.A.; Trifonov, R.E.; Popova, E.A. Medicinal chemistry of tetrazoles. Russ. Chem. Bull. Int. Ed. 2012, 61, 768–780. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.D.; Cook, J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Stöckigt, J.; Antonchick, A.P.; Wu, F.; Waldmann, H. The Pictet-Spengler Reaction in Nature and in Organic Chemistry. Angew. Chem. Int. Ed. 2011, 50, 8538–8564. [Google Scholar] [CrossRef] [PubMed]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound assisted green synthesis of bound type bis-heterocyclic carbazolyl imidazo [1,2-a]pyridines via Groebke-Blackburn-Bienayme reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]
- Claudio-Catalán, M.Á.; Pharande, S. G.; Quezada-Soto, A.; Kishore, K. G.; Rentería-Gómez, A.; Padilla-Vaca, F.; Gámez-Montaño, R. Solvent- and Catalyst-Free One-Pot Green Bound-Type Fused Bis-Heterocycles Synthesis via Groebke–Blackburn–Bienaymé Reaction/SNAr/Ring-Chain Azido-Tautomerization Strategy. ACS Omega 2018, 3, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Kaveti, B.; Ramírez-López, S.C.; Gámez Montaño, R. Ultrasound-assisted green one-pot synthesis of linked bis-heterocycle peptidomimetics via IMCR/post-transformation/tandem strategy. Tetrahedron Lett. 2018, 59, 4355–4358. [Google Scholar] [CrossRef]
- Shaabani, A.; Hooshmand, S.E. Diversity-oriented catalyst-free synthesis of pseudopeptides containing rhodanine scaffolds via a one-pot sequential isocyanide-based six-component reactions in water using ultrasound irradiation. Ultrason. Sonochem. 2018, 40, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pharande, S.G.; Corrales Escobosa, A.R.; Gámez-Montaño, R. Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction. Green Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]
- Chandgude, A.L.; Narducci, D.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Dömling, A. Diastereoselective one pot five-component reaction toward 4-(tetrazole)-1,3-oxazinanes. RSC Adv. 2017, 7, 49995–49998. [Google Scholar] [CrossRef] [PubMed]
- Unnamatla, M.V.B.; Islas-Jácome, A.; Quezada-Soto, A.; Ramírez-López, S.C.; Flores-Álamo, M.; Gámez-Montaño, R. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo[1,2-a]pyridin Tetrazolo[1,5-a]quinolines. J. Org. Chem. 2016, 81, 10576–10583. [Google Scholar] [CrossRef] [PubMed]
- Chandgude, A.L.; Dömling, A. An efficient Passerini tetrazole reaction (PT-3CR). Green Chem. 2016, 18, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, M.; Mahmoodi, N.O.; Shirini, F.; Azimi-Roshan, A.; Monfared, A. “On Water” Sonochemical Multicomponent Synthesis of Novel Bioactive 1,1′-(Aryl)bis(2-(cyclohexylamino)-2-oxoethane-1,1-diyl) Di(alkanoates and benzoates). J. Chem. 2013, 1–8. [Google Scholar] [CrossRef]
- Cárdenas-Galindo, L.E.; Islas-Jácome, A.; Alvarez-Rodríguez, N.V.; El Kaïm, L. Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a One-Pot Ugi-Azide/Pictet–Spengler Process. Synthesis 2014, 46, 49–56. [Google Scholar] [CrossRef][Green Version]
- Alvarez-Rodríguez, N.V.; Islas-Jácome, A.; Rentería-Gómez, A.; Cárdenas-Galindo, L.E.; Unnamatla, M.V.B.; Gámez-Montaño, R. Synthesis of 1′-tetrazolylmethyl-spiro[pyrrolidine-3,3′-oxindoles] via two coupled one-pot processes Ugi-azide/Pictet–Spengler and oxidative spiro-rearrangement. New J. Chem. 2018, 42, 1600–1603. [Google Scholar] [CrossRef]
- Gholamzadeh, P. The Pictet–Spengler Reaction: A Powerful Strategy for the Synthesis of Heterocycles. Adv. Heterocycl. Chem. 2019, 127, 153–226. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry a | Solvent b | Aditive c | T (°C) | Time (h) | Yield (%) d |
| 1 e | PhMe/MeOH | --- | rt USI | 3 | traces |
| 2 e | PhMe/MeOH | --- | 60 USI | 3 | traces |
| 3 f | PhMe/MeOH | --- | rt | 12 | 41 |
| 4 f | PhMe/MeOH | --- | rt USI | 1 | 45 |
| 5 f | PhMe/MeOH | --- | rt USI | 2 | 59 |
| 6 f | PhMe/MeOH | --- | 60 USI | 2 | 65 |
| 7 f | PhMe/MeOH | TFA | rt USI | 2 | 70 |
| 8 f | PhMe/MeOH | TFA | 60 USI | 2 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentería-Gómez, M.A.; Galindo, L.E.C.; Gámez-Montaño, R. Synthesis of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines via Ultrasound-Assisted One-Pot Ugi-azide/Pictet–Spengler Process. Proceedings 2019, 41, 67. https://doi.org/10.3390/ecsoc-23-06515
Rentería-Gómez MA, Galindo LEC, Gámez-Montaño R. Synthesis of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines via Ultrasound-Assisted One-Pot Ugi-azide/Pictet–Spengler Process. Proceedings. 2019; 41(1):67. https://doi.org/10.3390/ecsoc-23-06515
Chicago/Turabian StyleRentería-Gómez, Manuel A., Luis E. Cárdenas Galindo, and Rocío Gámez-Montaño. 2019. "Synthesis of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines via Ultrasound-Assisted One-Pot Ugi-azide/Pictet–Spengler Process" Proceedings 41, no. 1: 67. https://doi.org/10.3390/ecsoc-23-06515
APA StyleRentería-Gómez, M. A., Galindo, L. E. C., & Gámez-Montaño, R. (2019). Synthesis of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines via Ultrasound-Assisted One-Pot Ugi-azide/Pictet–Spengler Process. Proceedings, 41(1), 67. https://doi.org/10.3390/ecsoc-23-06515





