Thermophysical Characterization of TFSI Based Ionic Liquid and Lithium Salt Mixtures †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Products
2.2. Apparatus
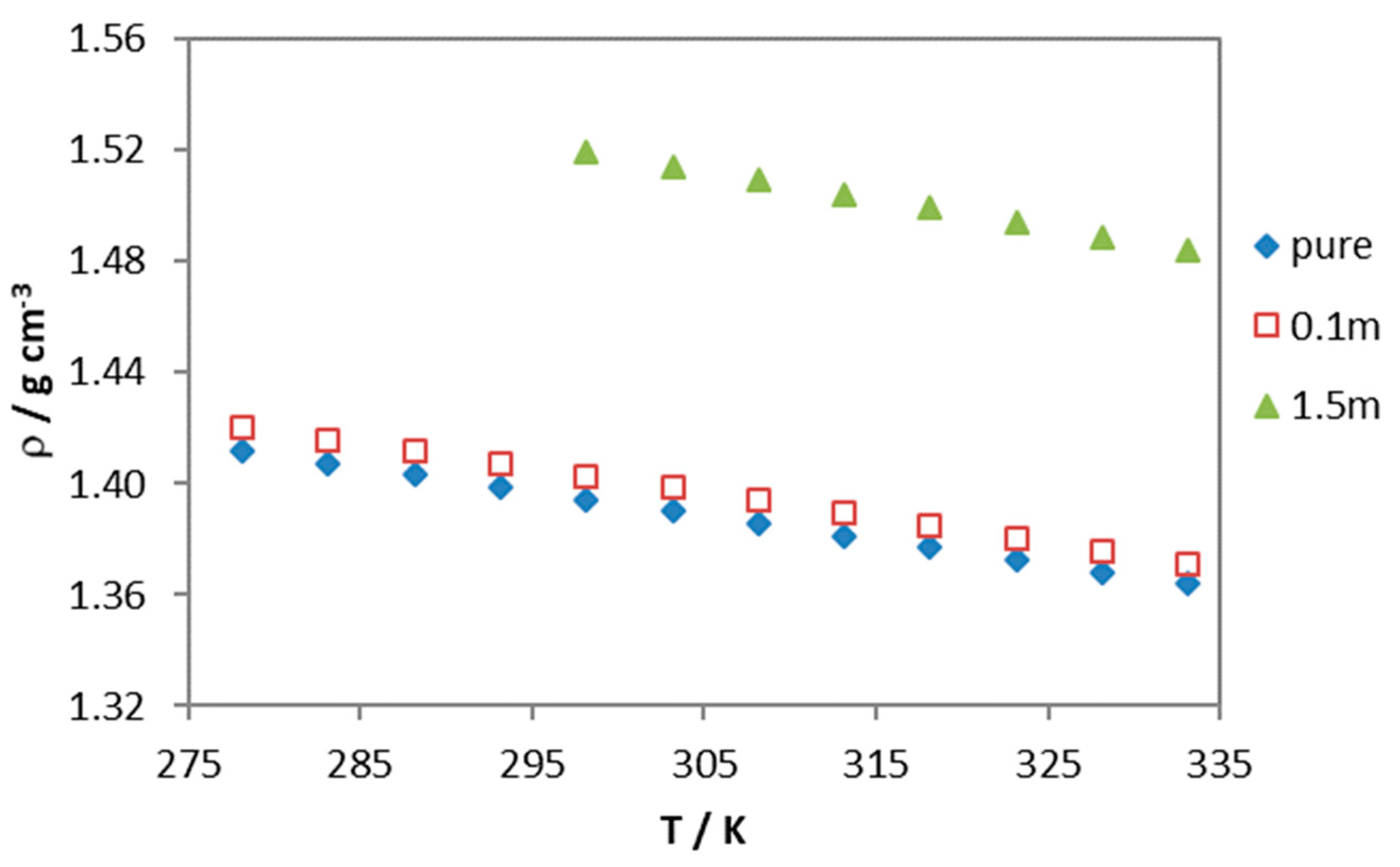
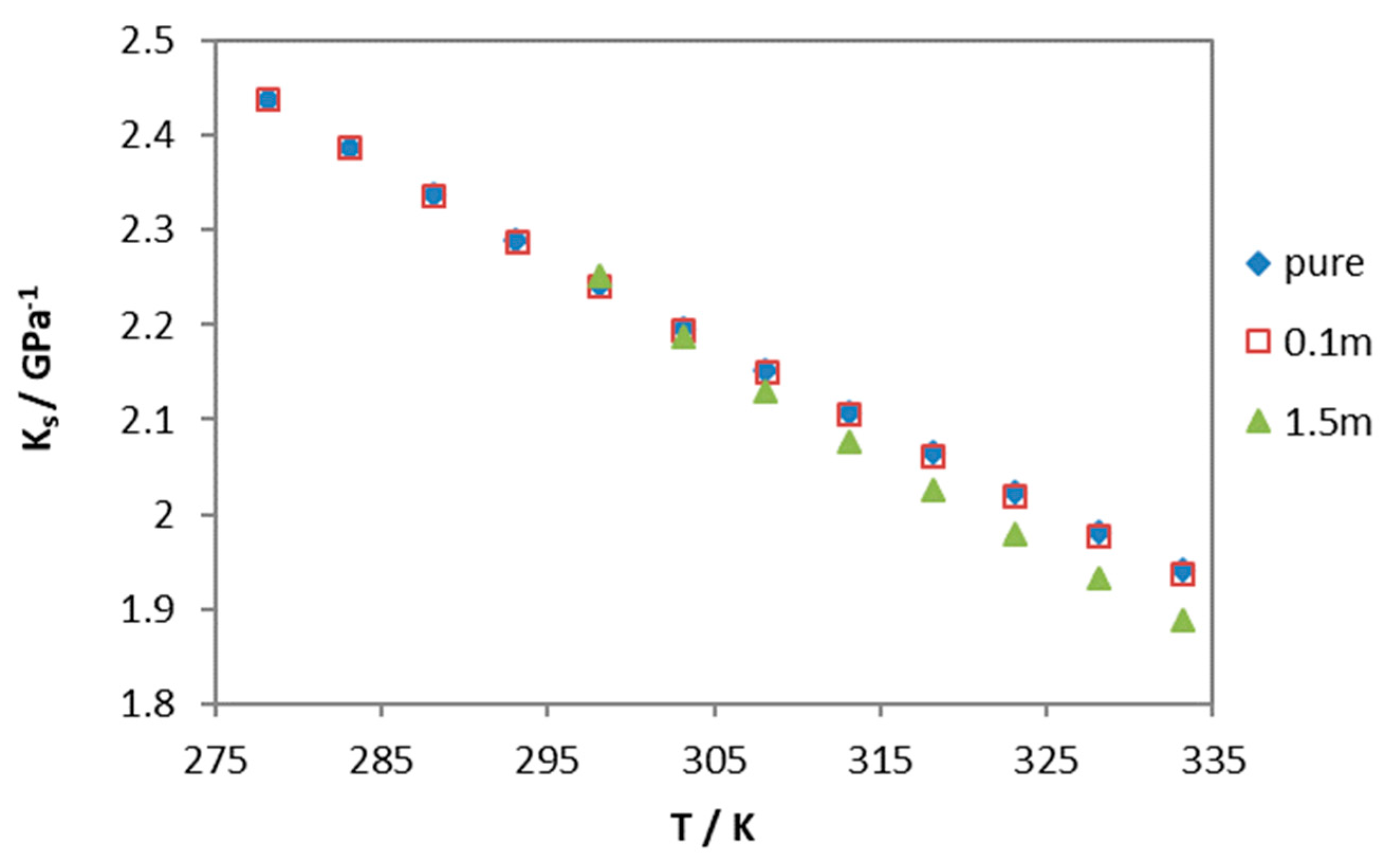
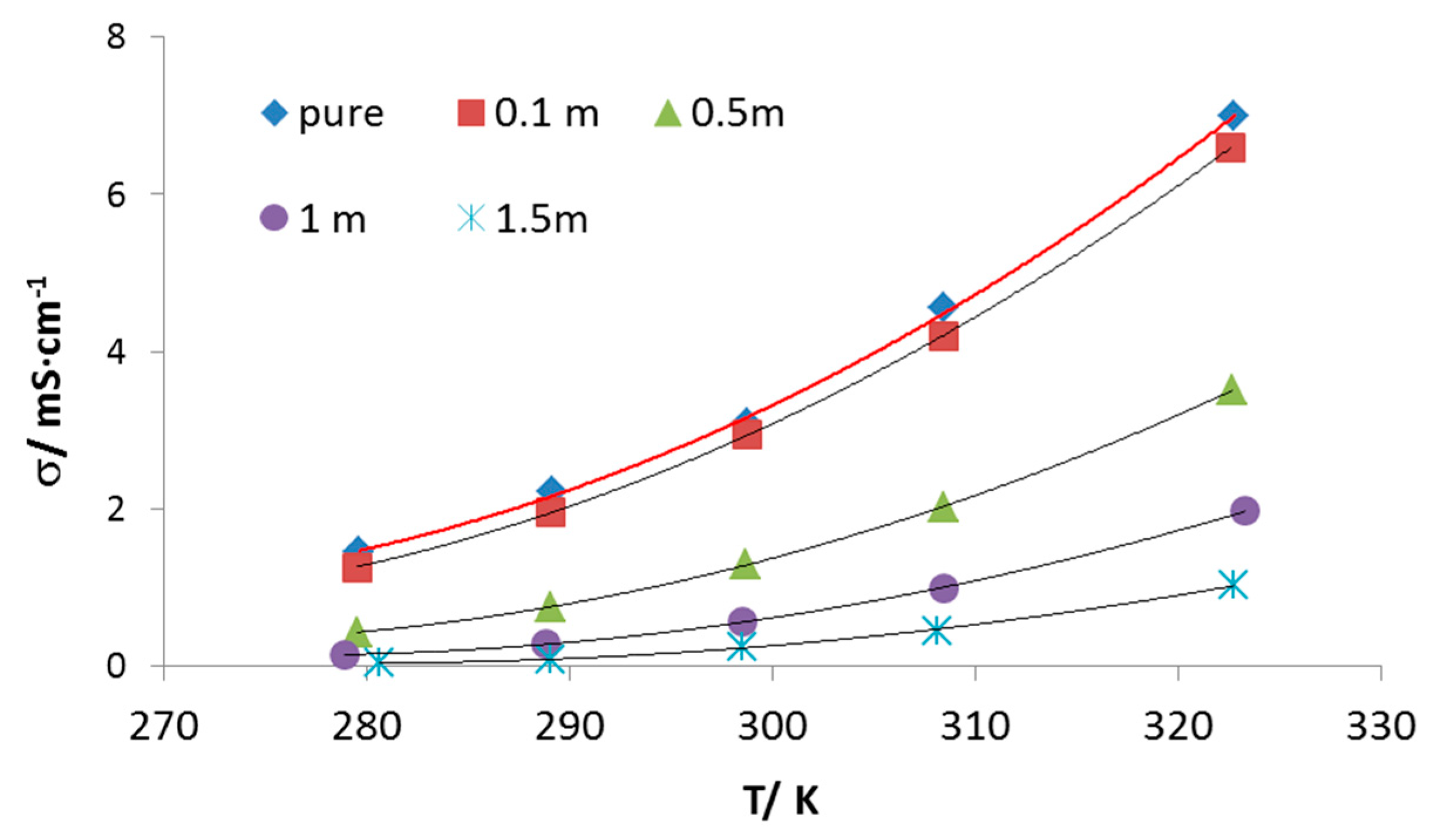
3. Results and Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Rogers, R.D.; Seddon, K.R. Ionic Liquids-Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Guimarey, M.J.G.; Salgado, M.R.; Comuñas, M.J.P.; López, E.R.; Amigo, A.; Cabaleiro, D.; Lugo, L.; Fernández, J. Effect of ZrO2 nanoparticles on thermophysical and rheological properties of three synthetic oils. J. Mol. Liq. 2018, 262, 126–138. [Google Scholar] [CrossRef]
- Mia, S.; Ohno, N. Relation between low temperature fluidity and sound velocity of lubricating oil. Tribol. Int. 2010, 43, 1043–1047. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Cabeza, O.; Varela, L.M. Liquid range of ionic liquid—Metal salt mixtures for electrochemical applications. J. Chem. Thermodyn. 2019, 134, 164–174. [Google Scholar] [CrossRef]
- Currás, M.R.; Vijande, J.; Piñeiro, M.M.; Lugo, L.; Salgado, J.; García, J. Behavior of the Environmentally Compatible Absorbent 1-Butyl-3-methylimidazolium Tetrafluoroborate with 2,2,2-Trifluoroethanol: Experimental Densities at High Pressures and Modeling of PVT and Phase Equilibria Behavior with PC-SAFT EoS. Ind. Eng. Chem. Res. 2011, 50, 4065–4076. [Google Scholar] [CrossRef]
- Neale, A.R.; Schütter, C.; Wilde, P.; Goodrich, P.; Hardacre, C.; Passerini, S.; Balducci, A.; Jacquemin, J. Physical—Chemical Characterization of Binary Mixtures of 1-Butyl-1-methylpyrrolidinium Bis {(trifluoromethyl) sulfonyl} imide and Aliphatic Nitrile Solvents as Potential Electrolytes for Electrochemical Energy Storage Applications. J. Chem. Eng. Data 2017, 62, 376–390. [Google Scholar] [CrossRef]
- Vranes, M.; Dozic, S.; Djeric, V.; Gadzuric, S. Physicochemical Characterization of 1-Butyl-3-methylimidazolium and 1-Butyl-1-methylpyrrolidinium Bis (trifluoromethylsulfonyl) imide. J. Chem. Eng. Data 2012, 57, 1072–1077. [Google Scholar] [CrossRef]
- Seoane, R.G.; Corderí, S.; Gómez, E.; Calvar, N.; González, E.J.; Macedo, E.A.; Domínguez, Á. Temperature Dependence and Structural Influence on the Thermophysical Properties of Eleven Commercial Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 2492–2504. [Google Scholar] [CrossRef]
- Zarrougui, R.; Dhahbi, M.; Lemordant, D. Effect of Temperature and Composition on the Transport and Thermodynamic Properties of Binary Mixtures of Ionic Liquid N-Butyl-N-methylpyrrolidinium bis (Trifluoromethanesulfonyl) imide and Propylene Carbonate. J. Solut. Chem. 2010, 39, 921–942. [Google Scholar] [CrossRef]
- Martinelli, A.; Matic, A.; Jacobsson, P.; Börjesson, L.; Fernicola, A.; Scrosati, B. Phase Behavior and Ionic Conductivity in Lithium Bis(trifluoromethanesulfonyl)imide-Doped Ionic Liquids of the Pyrrolidinium Cation and Bis (trifluoromethanesulfonyl) imide Anion. J. Phys. Chem. B 2009, 113, 11247–11251. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Miguez, L.; Vallet, P.; Tasende-Rodríguez, L.C.; Doval, A.; Villanueva, M.; Cabeza, Ó.; Varela, L.M.; Salgado, J.; Parajó, J.J. Thermophysical Characterization of TFSI Based Ionic Liquid and Lithium Salt Mixtures. Proceedings 2019, 41, 57. https://doi.org/10.3390/ecsoc-23-06618
Fernández-Miguez L, Vallet P, Tasende-Rodríguez LC, Doval A, Villanueva M, Cabeza Ó, Varela LM, Salgado J, Parajó JJ. Thermophysical Characterization of TFSI Based Ionic Liquid and Lithium Salt Mixtures. Proceedings. 2019; 41(1):57. https://doi.org/10.3390/ecsoc-23-06618
Chicago/Turabian StyleFernández-Miguez, Lois, Pablo Vallet, Lucia Camila Tasende-Rodríguez, Alejandro Doval, Maria Villanueva, Óscar Cabeza, Luis Miguel Varela, Josefa Salgado, and Juan José Parajó. 2019. "Thermophysical Characterization of TFSI Based Ionic Liquid and Lithium Salt Mixtures" Proceedings 41, no. 1: 57. https://doi.org/10.3390/ecsoc-23-06618
APA StyleFernández-Miguez, L., Vallet, P., Tasende-Rodríguez, L. C., Doval, A., Villanueva, M., Cabeza, Ó., Varela, L. M., Salgado, J., & Parajó, J. J. (2019). Thermophysical Characterization of TFSI Based Ionic Liquid and Lithium Salt Mixtures. Proceedings, 41(1), 57. https://doi.org/10.3390/ecsoc-23-06618




