Derivatives of boron-dipyrromethene (BODIPY) constitute an important class of chromophores. The BODIPY dyes can be applied in an extensive variety of applications, such as cellular imaging, photodynamic therapy, drug-delivery or organic photovoltaics [1,2,3,4]. BODIPYs are also excellent compounds to be applied as electron-donor materials in organic photovoltaic cells as they have a strong absorption in the visible spectra region, a photochemical and chemical stability, good solubility and a general suitable HOMO/LUMO frontier orbital energy levels (Figure 1).
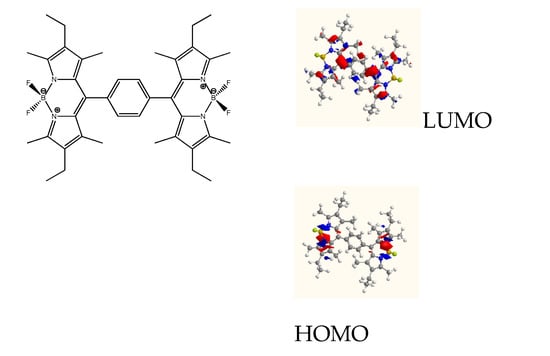
Figure 1.
Boron-dipyrromethene (BODIPY) (meso-phenyl-meso) dimer and its frontier molecular orbitals.
Following our work on the use of BODIPYs as photovoltaic materials [5], in this communication we present the synthesis and characterization of a meso-meso BODIPY dimer. This BODIPY dimer has all the main properties to work efficiently as electron-donor material since it exhibits strong absorbance in the visible spectrum, good solubility and suitable HOMO and LUMO energy orbitals to work as donor material in the bulky heterojunction BODIPY/PCBM layer, based solar cells.
Acknowledgments
The authors thank the ChemMat PhD program from the Fundação para a Ciência e Tecnologia (FCT, MEC, Portugal) for their general financial support (PD/BD/113702/2015) and to Centro de Química (UID/QUI/00313/2013) of University of Coimbra.
References
- Zhang, X.; Wang, C.; Jin, L.; Han, Z.; Xiao, Y. Photostable Bipolar Fluorescent Probe for Video Tracking Plasma Membranes Related Cellular Processes. ACS Appl. Mater. Interfaces 2014, 6, 12372–12379. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Bessette, A.; Hanan, G.S. Design, synthesis and photophysical studies of dipyrromethene-based materials: insights into their applications in organic photovoltaic devices. Chem. Soc. Rev. 2014, 43, 3342–3405. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.; Farinhas, J.; Da Silva, W.; Ghica, M.E.; Brett, C.M.; Morgado, J.; Sobral, A.J. Synthesis, characterization and application of meso-substituted fluorinated boron dipyrromethenes (BODIPYs) with different styryl groups in organic photovoltaic cells. Dye. Pigment. 2019, 168, 103–110. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).