Synthesis, Antibacterial, and Antifungal Activities of Hybrid Molecules Based on Alzheimer Disease Drugs and Bearing an Amino Acid Fragment †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
2.3. X-ray Crystallography
2.3.1. Sample Crystallization
2.3.2. Data Collection and Crystal Structure Refinement
2.3.3. Thermal Analyses
2.4. Biological Methods
- if xi > xk—the compound stimulates microorganisms to grow;
- if xi = xk—the compound does not affect the microorganisms;
- if xi = CFU/mL before the incubation—the compound has a bacteriostatic effect;
- if xi < xk—the compound has a bactericide effect.
3. Results and Discussion

3.1. X-ray Study
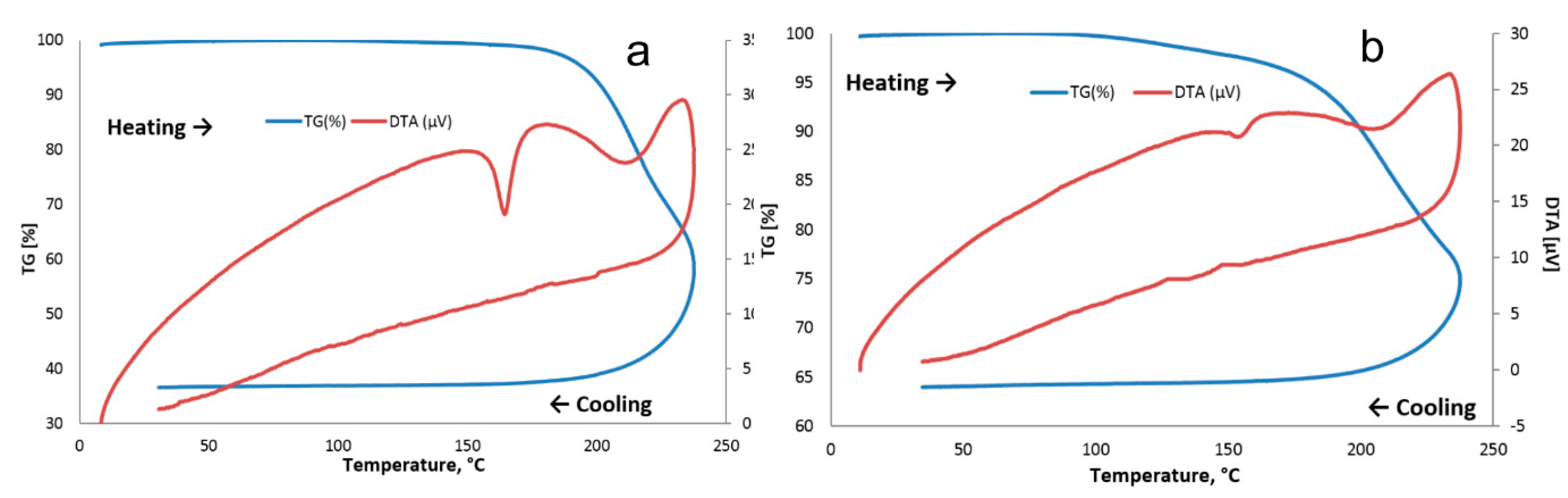
3.2. Thermal Behavior
3.3. Biological Part
3.3.1. Yeasts
3.3.2. Quantitative Analysis of Antimicrobial Activity: Determination of Minimum Inhibitory Concentration (MIC)
- ✓
- E. Coli—No. 3b > No. 3c, No. 3e;
- ✓
- S. enterica— No. 3b > No. 3e > No. 3c;
- ✓
- St. aureus— No. 3b > No. 3e > No. 3c;
- ✓
- B. megaterium— No. 3b > No. 3c > No. 3e;
- ✓
- St. epidermidis— No. 3b > No. 3c, No. 3e.
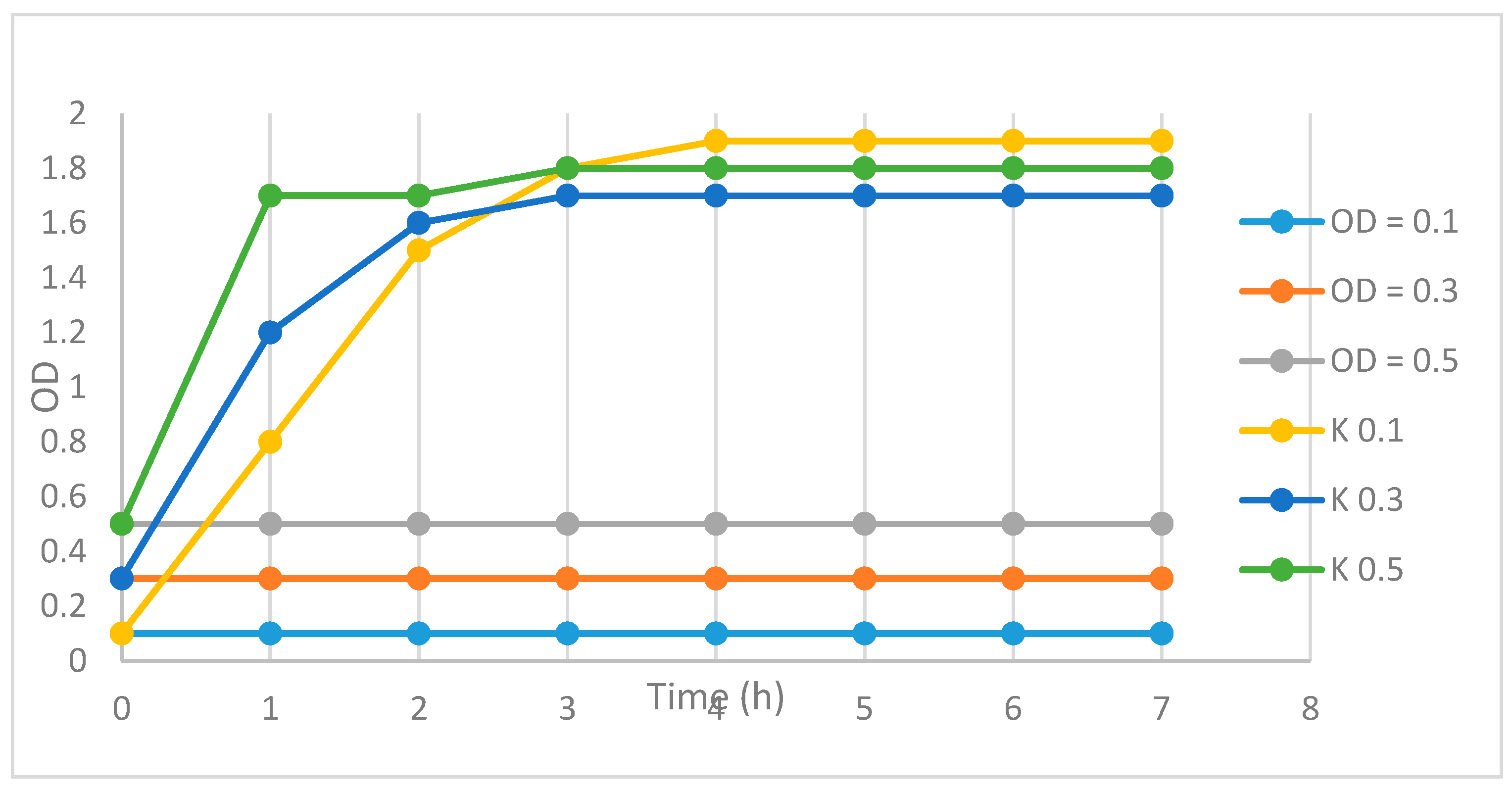
3.3.3. Time-Kill Test for Evaluation of Bacteriostatic/Bactericide Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aracava, Y.; Pereira, E.F.; Maelicke, A.; Albuquerque, E.X. Memantine blocks alpha7 nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J. Pharmacol. Exp. Ther. 2005, 312, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Li, D.; Hu, L.; Jin, S.; Yu, Y.; Cai, Z.; Shen, L. Atorvastatin Improves Plaque Stability in ApoE-Knockout Mice by Regulating Chemokines and Chemokine Receptors. PLoS ONE 2014, 9, e97009. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Zwolińska, K.; Leszek, J. The infectious etiology of Alzheimer’s diseas. J. Curr. Neuropharmacol. 2017, 15, 996–1009. [Google Scholar]
- The Infectious Etiology of Alzheimer’s Disease, Alzheimer’s Society Infections and Dementia. Available online: https://www.alzheimers.org.uk/about-dementia/risk-factors-and-prevention/infections-and-dementia (accessed on 25 October 2019).
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.M.; Novel, Y.H. Approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017, 133, 4–19. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 14, 742–750. [Google Scholar]
- Ibrahim, M.A.; Panda, S.S.; Birs, A.S.; Serrano, J.S.; Gonzalez, S.F.; Alamry, K.A.; Katritzky, A.R. Synthesis and antibacterial evaluation of amino acid-antibiotic conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.H. Antimicrobial drug developmentet he past, the present, and the future. Clin. Microb. Infec. Dis. 2004, 10, 23e31. [Google Scholar]
- CrysAlis PRO. Agilent Technologies; UK Ltd.: Yarnton, UK, 2011. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 7, 3–8. [Google Scholar]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storon, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystall. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Heatley, N.G. A method for the assay of penicillin. Biochem. J. 1944, 38, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S. Phage therapy pharmacology: Calculating phage dosing. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2011; Volume 77, pp. 1–40. [Google Scholar]
- Payne, R.J.; Jansen, V.A. Understanding bacteriophage therapy as a density-dependent kinetic process. J. Theor. Biol. 2001, 208, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
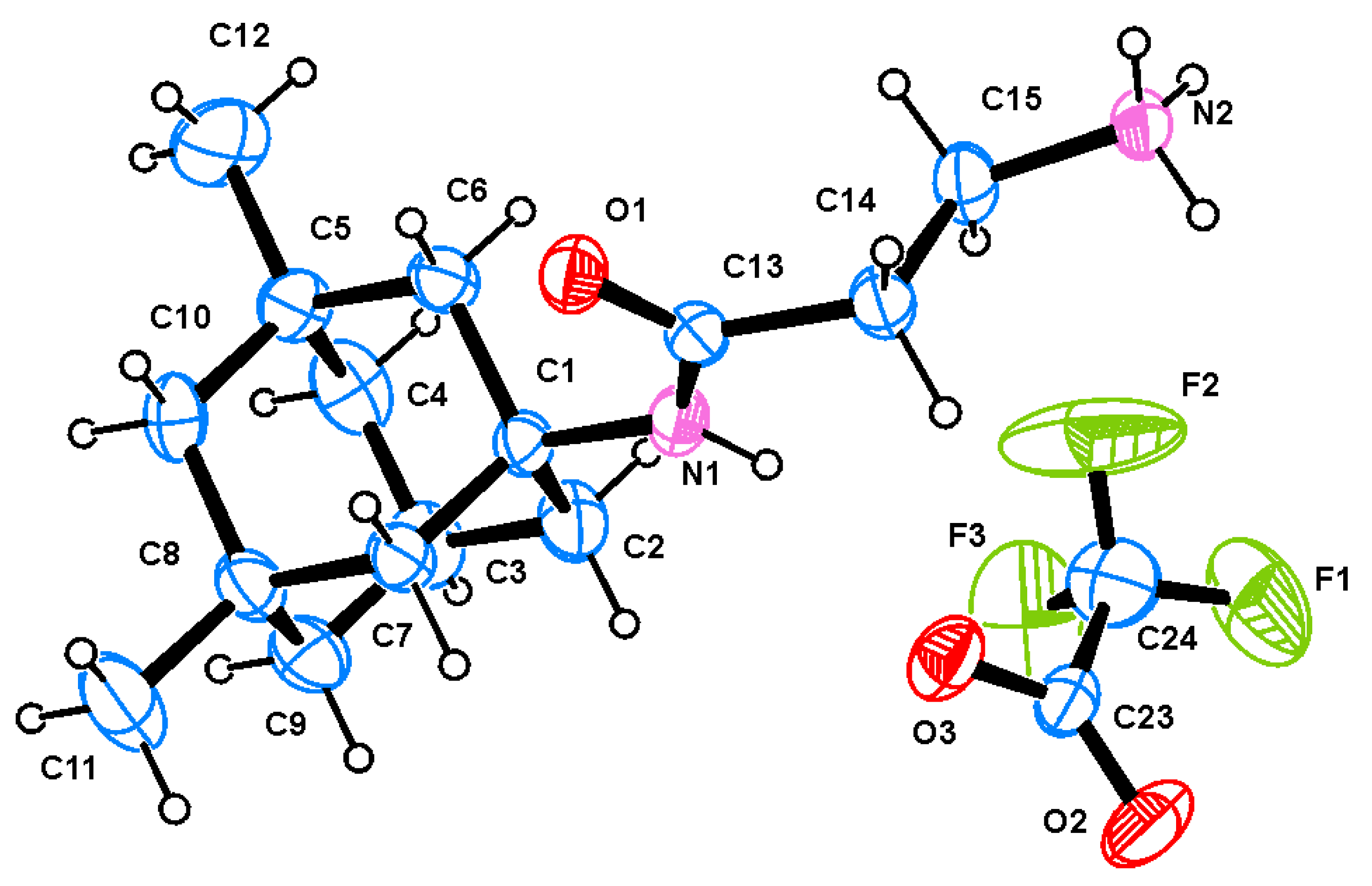

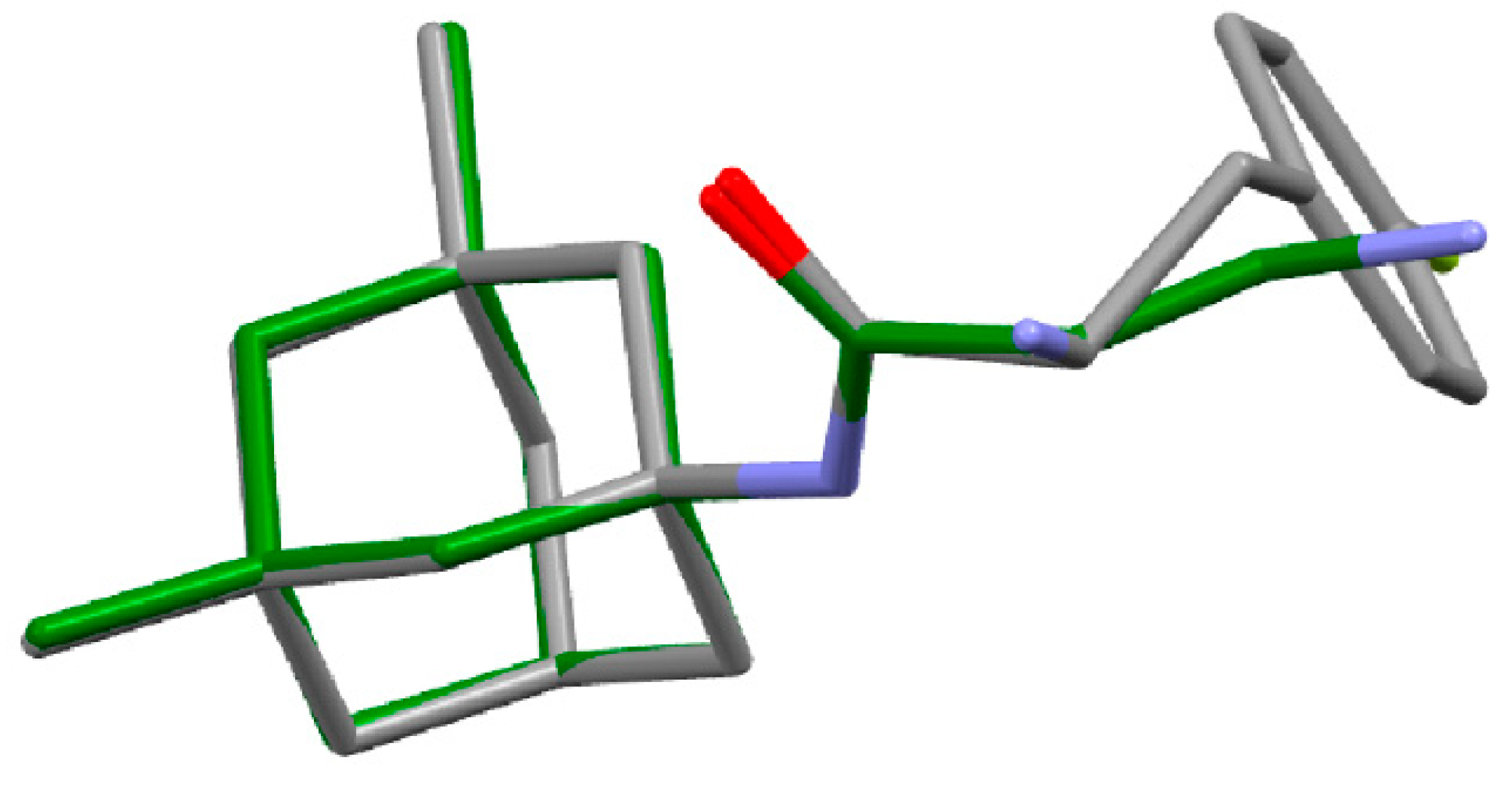
| Bond | Length [Å] for β-Ala-Memantine | Length [Å] for 4-F-Phe-Memantine |
|---|---|---|
| O1–C13 | 1.219(5) | 1.229(7) |
| N1–C1 | 1.485(5) | 1.485(9) |
| N1–C13 | 1.336(6) | 1.312(8) |
| C13–C14 | 1.513(6) | 1.522(9) |
| C15–C14 | 1.478(6) | 1.529(9) |
| C1–C7 | 1.523(6) | 1.500(10) |
| C1–C2 | 1.523(7) | 1.538(11) |
| C1–C6 | 1.516(6) | 1.520(11) |
| C2–C3 | 1.534(7) | 1.547(14) |
| C4–C3 | 1.517(9) | 1.530(2) |
| C3–C9 | 1.502(9) | 1.500(2) |
| C5–C4 | 1.524(8) | 1.531(17) |
| C5–C12 | 1.529(8) | 1.517(16) |
| C7–C8 | 1.538(6) | 1.520(11) |
| C10–C8 | 1.527(7) | 1.508(12) |
| C11–C8 | 1.539(8) | 1.544(15) |
| Angle | Angle [°] for β-Ala-Memantine | Angle [°] for 4-F-Phe-Memantine |
|---|---|---|
| C13–N1–C1 | 126.6(4) | 128.0(5) |
| O1–C13–N1 | 123.4(4) | 126.3(6) |
| O1–C13–C14 | 120.8(4) | 118.0(6) |
| N1–C13–C14 | 115.7(4) | 115.6(5) |
| N1–C1–C7 | 110.3(4) | 112.1(6) |
| N1–C1–C2 | 107.3(4) | 104.7(6) |
| N1–C1–C6 | 110.8(4) | 111.8(6) |
| D- | H | A | d(D-H)/Å | d(H...A)/Å | d(D...A)/Å | D-H...A/° |
|---|---|---|---|---|---|---|
| N2- | H2A | O11 | 0.89 | 1.87 | 2.725(5) | 161.1 |
| N2- | H2B | O152 | 0.89 | 1.92 | 2.725(5) | 149.3 |
| N2- | H2C | O43 | 0.89 | 1.93 | 2.788(5) | 162.3 |
| N3- | H3 | O4 | 0.86 | 2.12 | 2.908(5) | 151.5 |
| C6- | H6A | O1 | 0.97 | 2.61 | 3.169(6) | 117 |
| C8- | H8B | F2 | 0.97 | 2.56 | 3.467(9) | 155.9 |
| C14- | H14B | O1 | 0.97 | 2.45 | 3.047(6) | 119.5 |
| (A) β-Alanine-Memantine. | ||||||
| D- | H | A | d(D-H)/Å | d(H...A)/Å | d(D...A)/Å | D-H...A/° |
| N2- | H2A | O111 | 0.89 | 2.15 | 2.916(9) | 144.3 |
| N2- | H2B | O82 | 0.89 | 1.91 | 2.766(8) | 161.2 |
| N2- | H2C | O8 | 0.89 | 2.04 | 2.868(10) | 153.9 |
| N3- | H3 | O41 | 0.86 | 2.1 | 2.942(8) | 164.5 |
| C7- | H7 | O41 | 0.98 | 2.57 | 3.441(9) | 147.3 |
| C23- | H23B | O1 | 0.97 | 2.5 | 3.071(10) | 117.8 |
| (B) 4-F-Phenilalanine-Memantine. | ||||||
| Compound No. (10 mM) | Gram (−) Bacteria | Gram (+) Bacteria | |||
|---|---|---|---|---|---|
| S. enterica | E. coli | St. aureus | B. megaterium | St. epidermidis | |
| Gly-memantine | n/a | n/a | n/a | n/a | n/a |
| 4-F-Phe memantine | 20 | 22 | 21 | 22 | 24 |
| Val-memantine | 11 | 11 | n/a | 16 | n/a |
| β-Ala-memantine | 16 | n/a | n/a | n/a | n/a |
| Thz-memantine | n/a | 13 | 10 | 16 | n/a |
| Fmoc-Thz-Thz-memantine | n/a | n/a | n/a | n/a | n/a |
| Memantine.HCl | n/a | n/a | n/a | n/a | n/a |
| 4-F-L-Phenylalanine | n/a | n/a | n/a | n/a | n/a |
| DMSO | n/a | n/a | n/a | n/a | n/a |
| Tetracycline | 26 | 26 | 28 | 34 | n/a |
| Compound № (10 mM) | Rhodotorula sp. | Candida lusitaniae | Sacch. cerevisiae |
|---|---|---|---|
| Gly-memantine | n/a | n/a | 14 |
| 4-F-Phe-memantine | 26 | 23 | 21 |
| Val-memantine | n/a | n/a | n/a |
| β-Ala-memantine | n/a | n/a | n/a |
| Thz-memantine | n/a | n/a | n/a |
| Fmoc-Thz-Thz-memantine | n/a | n/a | n/a |
| Memantine.HCl | n/a | n/a | n/a |
| 4-F-L-Phenylalanine | n/a | n/a | n/a |
| DMSO | n/a | n/a | n/a |
| Nystatin | 26 | 24 | n/a |
| Compound No. (µM) | Gram (−) Bacteria | Gram (+) Bacteria | Yeasts | |||
|---|---|---|---|---|---|---|
| S. enterica | E. coli | St. aureus | B. megaterium | Rhodotorula sp. 16-25 | Candida lusitaniae 74-4 | |
| Gly-mem (3a) | 1.25 | n/a | n/a | 1.25 | n/a | 0.3125 |
| 4-F-Phe mem (3b) | 0.156 | 1.25 | 1.25 | 0.625 | 0.078 | 0.078 |
| Val-mem (3c) | 1.25 | 5 | 10 | 2.5 | n/a | 0.3125 |
| β-Ala-mem (3d) | n/a | 10 | n/a | n/a | n/a | 0.078 |
| Thz-mem (3e) | 5 | 5 | 10 | 10 | 1.25 | 0.3125 |
| Hours | Penicillium chaviforme d (mm) | Fusarium graminearum d (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| К | Topsin | DMSO | 3b | К | Topsin | DMSO | 3b | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 5, 6 | 0/0 | 9, 8 | 7, 6 | 10/7 | 15/16 | 20/22 | 7, 7 |
| 48 | 11, 12 | 0/0 | 14/13 | 11, 10 | 26/26 | 39/39 | 39/39 | 35/35 |
| 72 | 16/16 | 0/0 | 13/14 | 15/14 | 52/49 | 44/44 | 70/62 | 43/49 |
| 98 | 21/21 | 0/0 | 23/23 | 20/19 | 71/70 | 49/45 | 83/82 | 46/50 |
| 120 | 25/25 | 0/0 | 27/27 | 24/22 | 85/85 | 50/50 | 85/85 | 51/55 |
| Kr (mm/h) | 19, 7 | 0 | 17, 5 | 15, 7 | 84, 9 | 49, 8 | 84, 8 | 19, 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chayrov, R.; Tencheva, A.; Sbirkova-Dimitrova, H.; Shivachev, B.; Kujumdzieva, A.; Nedeva, T.; Stankova, I. Synthesis, Antibacterial, and Antifungal Activities of Hybrid Molecules Based on Alzheimer Disease Drugs and Bearing an Amino Acid Fragment. Proceedings 2019, 41, 23. https://doi.org/10.3390/ecsoc-23-06602
Chayrov R, Tencheva A, Sbirkova-Dimitrova H, Shivachev B, Kujumdzieva A, Nedeva T, Stankova I. Synthesis, Antibacterial, and Antifungal Activities of Hybrid Molecules Based on Alzheimer Disease Drugs and Bearing an Amino Acid Fragment. Proceedings. 2019; 41(1):23. https://doi.org/10.3390/ecsoc-23-06602
Chicago/Turabian StyleChayrov, Radoslav, Aleksandra Tencheva, Hristina Sbirkova-Dimitrova, Boris Shivachev, Anna Kujumdzieva, Trayana Nedeva, and Ivanka Stankova. 2019. "Synthesis, Antibacterial, and Antifungal Activities of Hybrid Molecules Based on Alzheimer Disease Drugs and Bearing an Amino Acid Fragment" Proceedings 41, no. 1: 23. https://doi.org/10.3390/ecsoc-23-06602
APA StyleChayrov, R., Tencheva, A., Sbirkova-Dimitrova, H., Shivachev, B., Kujumdzieva, A., Nedeva, T., & Stankova, I. (2019). Synthesis, Antibacterial, and Antifungal Activities of Hybrid Molecules Based on Alzheimer Disease Drugs and Bearing an Amino Acid Fragment. Proceedings, 41(1), 23. https://doi.org/10.3390/ecsoc-23-06602





