New 1,3,5-Triazine Derivatives Incorporating Aminobenzenesulfonamide, Aminoalcohol, Piperazine, Chalcone or Stilbene Structural Motifs and Evaluation of Their Antioxidative Activity †
Abstract
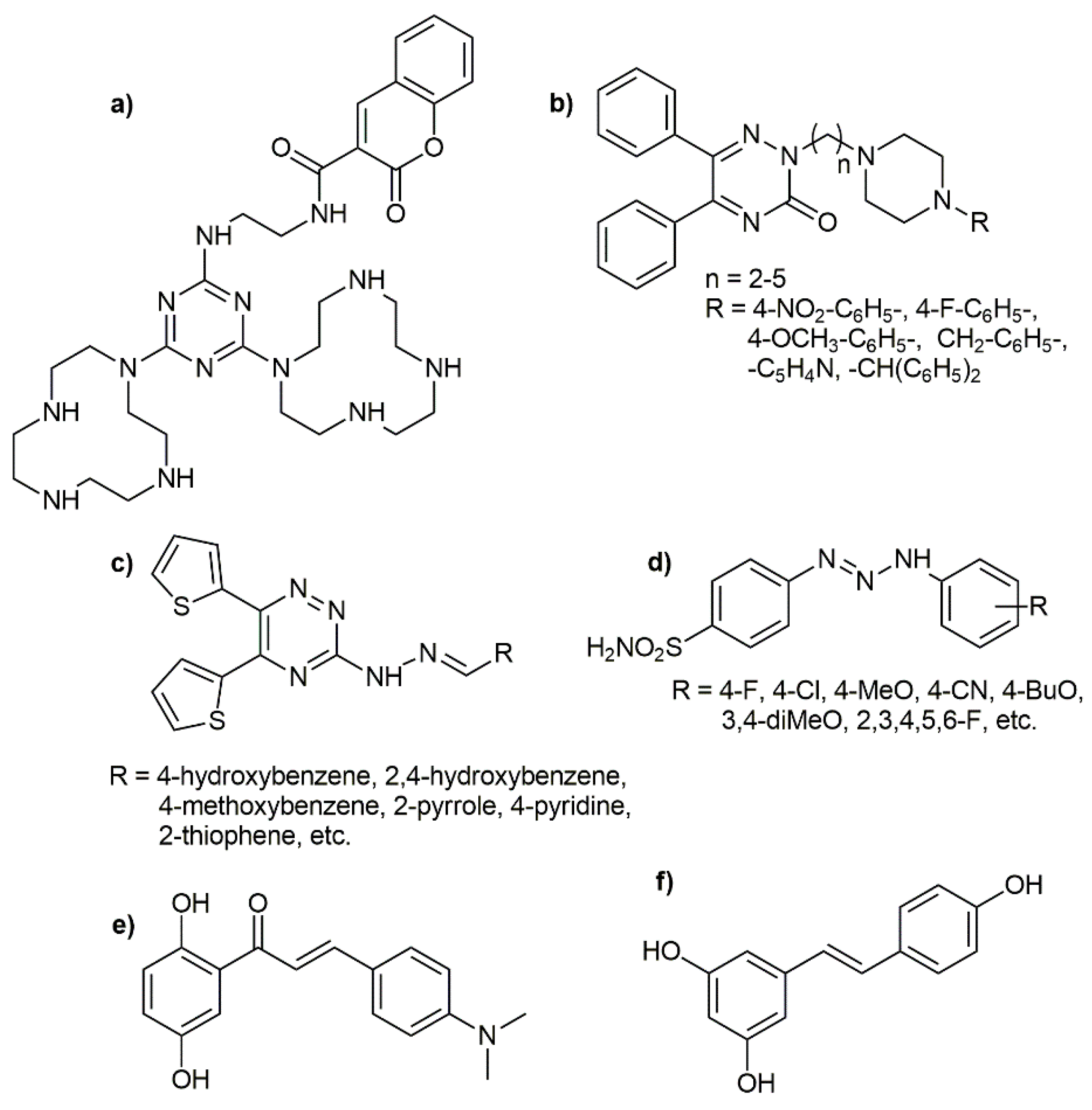
:1. Introduction
2. Materials and Methods
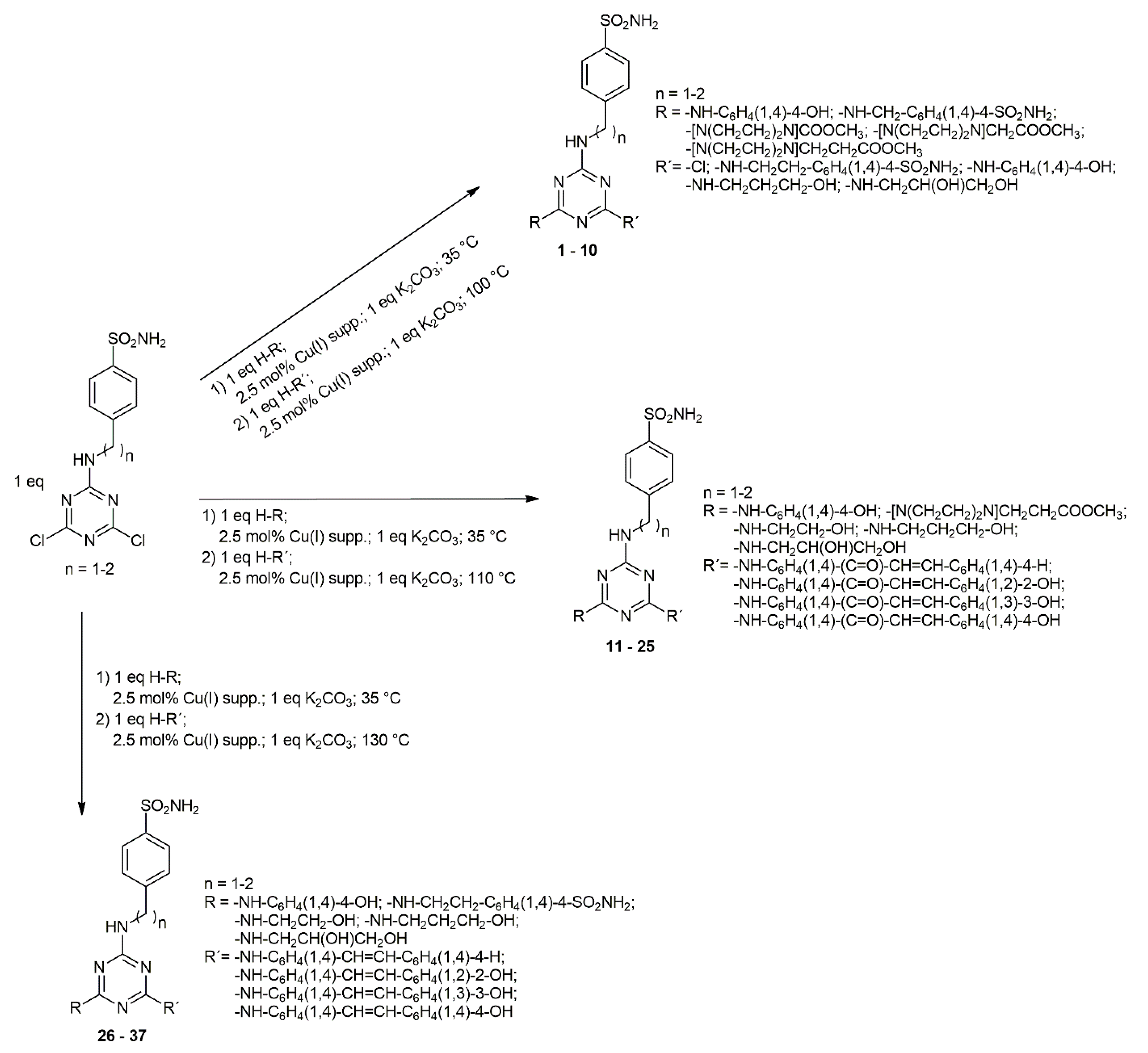
2.1. Chemistry
2.2. Characterization of New Compounds
- (E)-4-[2-({4-[(2-Hydroxyethyl)amino]-6-({4-[3-(3-hydroxyphenyl)acryloyl]phenyl}amino)-1,3,5-triazin-2-yl}amino)ethyl]benzenesulfonamide (19): 77.7%; brown solid; m.p. 256–258 °C; 1H-NMR (500 MHz, DMSO-d6) δ ppm 7.91 (2H, d, J = 8.1 Hz, CH), 7.80 (2H, d, J = 8.1 Hz, CH), 7.71 (1H, d, J = 15.5 Hz, CH=CH-CO), 7.56 (2H, d, J = 8.1 Hz, CH), 7.48 (1H, d, J = 15.5 Hz, CH=CH-CO), 7.26 (2H, d, J = 8.1 Hz, CH), 6.91 (2H, d, J = 8.1 Hz, CH), 6.69 (7H, s, OH, NH, NH2), 4.56–4.52 (2H, m, CH2), 3.55–3.53 (2H, m, CH2-OH), 3.34–3.32 (2H, m, NH-CH2), 2.93–2.91 (2H, m, CH2); 13C-NMR (125 MHz, DMSO-d6) δ ppm 189.4, 168.6, 166.3, 165.6, 146.7, 144.5, 144.1, 142.3, 141.8, 141.2, 138.2, 130.4, 128.2, 126.6, 125.9, 119.7, 118.6, 114.2, 58.8, 44.5, 41.9, 36.6; IR νmax (cm−1) 3336, 3222 (OH, NH, NH2), 2930 (CHalif, CH2alif), 1698, 1660, 1654, 1635 (C=C, C=N, C=O), 1583, 1345, 1155, (SO2NH2), 1076 (C-OH), 1034 (COC)
- (E)-Methyl 3-(4-{4-({4-[3-(4-hydroxyphenyl) acryloyl]phenyl}amino)-6-[(4-sulfamoyl-phenethyl)amino] -1,3,5-triazin-2-yl}piperazin-1-yl)propanoate (25): 78.1%; orange solid; m.p. 101–103 °C; 1H-NMR (500 MHz, DMSO-d6) δ ppm 7.97 (2H, d, J = 8.1 Hz, CH), 7.94 (2H, d, J = 8.1 Hz, CH), 7.8 (1H, d, J = 15.5 Hz, CH=CH-CO), 7.42 (1H, d, J = 15.5 Hz, CH=CH-CO), 7.31 (2H, d, J = 8.1 Hz, CH), 7.28 (2H, d, J = 8.1 Hz, CH), 6.99 (2H, d, J = 8.1 Hz, CH), 6.68 (2H, d, J = 8.1 Hz, CH), 3.91–3.86 (4H, m, CH2), 3.74–3.71 (2H, m, NH-CH2), 3.65 (3H, s, CH3), 2.99–2.87 (4H, m, CH2), 2.76–2.74 (2H, m, CH2), 2.59 (2H, t, J = 7.8 Hz, N-CH2), 2.11 (2H, t, J = 7.8 Hz, CH2-COO); 13C-NMR (125 MHz, DMSO-d6) δ ppm 186.7, 171.9, 167.8, 167.1, 162.6, 159.9, 158.4, 154.2, 150.9, 143.8, 142.7, 144.3, 119.2, 131.3, 130.6, 126.8, 124.9, 116.3, 113.1, 66.4, 51.3, 51.1, 44.3, 43.7, 35.1, 31.6; IR νmax (cm−1) 3332, 3218 (OH, NH, NH2), 2950 (CHalif, CH2alif) 1698, 1683, 1669, 1651, 1646, 1630, 1588 (C=C, C=N, COO), 1349 (SO2NH2), 1227 (OH), 1162 (SO2NH2), 1105, 1028 (COC)
2.3. Determination of Antioxidant Activity by ABTS Method
3. Results and Discussion
| Compound | n | R1 | R2 | 1 × 10−2 M; (%) 2 | 1 × 10−4 M; (%) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 5 min | 30 min | 60 min | 0 min | 5 min | 30 min | 60 min | ||||
| 1 1 | 1 | NH-C6H4(1,4)-4-OH | Cl | 92.05 | 92.00 | 91.88 | 91.88 | 31.52 | 38.51 | 44.71 | 73.51 |
| 2 1 | 2 | NH-C6H4(1,4)-4-OH | Cl | 91.38 | 91.26 | 91.21 | 91.21 | 45.38 | 49.84 | 63.65 | 73.44 |
| 3 1 | 1 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 35.58 | 47.98 | 58.35 | 66.69 | 21.82 | 24.81 | 27.23 | 35.57 |
| 4 1 | 2 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 51.53 | 74.64 | 79.54 | 90.36 | 31.46 | 35.24 | 40.99 | 41.83 |
| 5 1 | 1 | [N(CH2CH2)2N]COOCH3 | NH-CH2CH(OH)CH2OH | 90.70 | 92.84 | 92.95 | 92.90 | 24.02 | 31.01 | 39.92 | 52.99 |
| 6 1 | 1 | [N(CH2CH2)2N]CH2COOCH3 | NH-CH2CH(OH)CH2OH | 63.98 | 82.13 | 87.15 | 91.77 | 25.37 | 35.07 | 34.73 | 50.06 |
| 7 1 | 1 | [N(CH2CH2)2N]CH2COOCH3 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 79.20 | 86.47 | 88.33 | 88.39 | 24.81 | 34.28 | 42.90 | 64.38 |
| 8 1 | 1 | [N(CH2CH2)2N]CH2CH2COOCH3 | NH-C6H4(1,4)-4-OH | 89.63 | 89.69 | 89.69 | 89.69 | 38.28 | 39.41 | 41.89 | 60.66 |
| 9 1 | 1 | NH-C6H4(1,4)-4-OH | NH-CH2CH(OH)CH2OH | 89.40 | 89.29 | 89.18 | 89.12 | 33.55 | 36.31 | 48.71 | 78.24 |
| 10 1 | 1 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2CH2OH | 43.81 | 62.80 | 75.54 | 82.19 | 26.39 | 29.09 | 30.67 | 49.16 |
| 11 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-H | 52.03 | 68.04 | 75.88 | 81.57 | 24.36 | 23.68 | 25.88 | 42.62 |
| 12 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-H | 52.60 | 70.01 | 85.29 | 89.12 | 18.33 | 23.97 | 31.63 | 46.34 |
| 13 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 89.57 | 89.57 | 89.63 | 89.63 | 24.70 | 31.91 | 34.62 | 50.79 |
| 14 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 90.47 | 90.59 | 90.25 | 90.14 | 19.01 | 18.55 | 23.18 | 38.39 |
| 15 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 90.70 | 90.36 | 89.97 | 89.74 | 34.28 | 35.41 | 37.32 | 57.56 |
| 16 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 91.43 | 91.32 | 91.04 | 90.76 | 23.46 | 25.04 | 24.13 | 40.03 |
| 17 | 1 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 90.59 | 90.19 | 89.80 | 89.52 | 24.02 | 28.87 | 30.90 | 49.39 |
| 18 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 90.08 | 89.40 | 88.84 | 89.07 | 22.50 | 23.63 | 24.70 | 47.07 |
| 19 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 89.12 | 89.01 | 89.01 | 89.12 | 25.49 | 34.79 | 47.81 | 65.28 |
| 20 | 2 | NH-CH2CH(OH)CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 91.15 | 91.04 | 90.76 | 90.53 | 36.25 | 38.85 | 40.59 | 58.97 |
| 21 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-H | 91.21 | 91.15 | 91.15 | 91.09 | 22.56 | 22.61 | 21.32 | 39.07 |
| 22 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-OH | 89.85 | 88.61 | 89.23 | 90.76 | 22.22 | 22.89 | 23.46 | 42.73 |
| 23 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 90.53 | 90.42 | 90.31 | 90.31 | 25.60 | 27.57 | 32.31 | 36.31 |
| 24 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 89.40 | 87.32 | 88.56 | 90.19 | 37.72 | 39.47 | 42.28 | 69.34 |
| 25 | 2 | [N(CH2CH2)2N]CH2CH2COOCH3 | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-OH | 90.47 | 91.83 | 91.77 | 92.11 | 40.03 | 46.96 | 55.25 | 87.09 |
| 26 | 1 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-H | 39.92 | 59.70 | 81.96 | 89.40 | 16.92 | 16.53 | 18.78 | 38.51 |
| 27 | 1 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 88.67 | 89.97 | 90.19 | 90.25 | 24.08 | 27.46 | 32.65 | 43.41 |
| 28 | 1 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-OH | 75.43 | 79.31 | 83.99 | 87.37 | 26.61 | 26.84 | 28.19 | 41.95 |
| 29 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 55.92 | 65.05 | 74.47 | 82.92 | 16.69 | 21.71 | 28.98 | 39.63 |
| 30 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 83.37 | 90.19 | 91.66 | 91.71 | 1.70 | 3.67 | 7.23 | 43.19 |
| 31 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-OH | 79.20 | 85.91 | 88.33 | 88.39 | 16.98 | 18.22 | 21.99 | 30.73 |
| 32 | 2 | NH-CH2CH(OH)CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 36.37 | 56.15 | 76.89 | 85.74 | 14.95 | 15.12 | 23.80 | 59.64 |
| 33 | 2 | NH-CH2CH(OH)CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-OH | 52.54 | 55.98 | 76.72 | 85.12 | 16.92 | 17.31 | 24.36 | 48.65 |
| 34 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-H | 90.36 | 90.31 | 90.02 | 89.85 | 18.27 | 19.34 | 27.40 | 48.20 |
| 35 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-2-OH | 71.03 | 75.09 | 81.96 | 87.83 | 18.78 | 19.63 | 23.68 | 44.14 |
| 36 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 90.08 | 89.91 | 89.80 | 89.57 | 28.53 | 31.97 | 34.28 | 48.26 |
| 37 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-OH | 91.04 | 90.98 | 90.81 | 90.64 | 23.51 | 25.21 | 32.31 | 29.55 |
| Trolox | - | - | - | 89.29 | 89.23 | 88.18 | 89.23 | 34.96 | 35.41 | 37.61 | 41.49 |
| Ascorbic acid | - | - | - | 88.33 | 88.45 | 88.33 | 88.45 | 29.09 | 28.81 | 29.83 | 31.07 |
| Compound | n | R1 | R2 | IC50 (μM) 2 | |
|---|---|---|---|---|---|
| 0 min | 60 min | ||||
| 1 1 | 1 | NH-C6H4(1,4)-4-OH | Cl | 103.89 | 26.59 |
| 2 1 | 2 | NH-C6H4(1,4)-4-OH | Cl | 107.20 | 17.16 |
| 7 1 | 1 | [N(CH2CH2)2N]CH2COOCH3 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 317.78 | 43.84 |
| 8 1 | 1 | [N(CH2CH2)2N]CH2CH2COOCH3 | NH-C6H4(1,4)-4-OH | 180.98 | 51.47 |
| 9 1 | 1 | NH-C6H4(1,4)-4-OH | NH-CH2CH(OH)CH2OH | 127.98 | 27.78 |
| 18 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 1800.00 | 153.70 |
| 19 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 288.06 | 20.16 |
| 24 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 147.43 | 45.78 |
| 25 | 2 | [N(CH2CH2)2N]CH2CH2COOCH3 | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-OH | 53.72 | 17.22 |
| Trolox | - | - | - | 293.46 | 178.33 |
| Ascorbic acid | - | - | - | 169.24 | 147.47 |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative Stress in Alzheimer’s Disease: Why Did Antioxidant Therapy Fail? Oxidative Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Pota, K.; Turan, L.S.; da Costa, V.C.P.; Akkaraju, G.; Green, K.N. Synthesis, Characterization, and Activity of a Triazine Bridged Antioxidant Small Molecule. ACS Chem. Neurosci. 2017, 8, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Sisein, E.A. Biochemistry of free radicals and antioxidants. Sch. Acad. J. Biosci. 2014, 2, 110–118. [Google Scholar]
- Kumar, S. The importance of antioxidant and their role in pharmaceutical science—A review. Asian. J. Med. Chem. Pharm. Sci. 2014, 1, 27–44. [Google Scholar]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maulik, N.; McFadden, D.; Otani, H.; Thirunavukkarasu, M.; Parinandi, N.L. Antioxidants in Longevity and Medicine. Oxidative Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Toda, S. Polyphenol Content and Antioxidant Effects in Herb Teas. Chin. Med. 2011, 2, 29–31. [Google Scholar] [CrossRef]
- Tripathi, P.N.; Srivastava, P.; Sharma, P.; Tripathi, M.K.; Seth, A.; Tripathi, A.; Rai, S.N.; Singh, S.P.; Shrivastava, S.K. Biphenyl-3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg. Chem. 2019, 85, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Firuzi, O.; Khoshneviszadeh, M.; Nadri, H.; Edraki, N.; Miri, R. Synthesis and structure-activity relationship study of multi-target triazine derivatives as innovative candidates for treatment of Alzheimer’s disease. Bioorg. Chem. 2018, 77, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Boga, M.; Lolak, N.; Tuneg, M.; Sanku, R.K.K. Design, synthesis and biological evaluation of 1,3-diaryltriazene-substituted sulfonamides as antioxidant, acetylcholinesterase and butyrylcholinesterase inhibitors. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Narsinghani, T.; Sharma, M.C.; Bhargav, S. Synthesis, docking studies and antioxidant activity of some chalcone and aurone derivatives. Med. Chem. Res. 2013, 22, 4059–4068. [Google Scholar] [CrossRef]
- San Miguel-Chávez, R. Phenolic Antioxidant Capacity: A Review of the State of the Art. In Phenolic Compounds—Biological Activity; IntechOpen: London, UK, 2017; pp. 59–74. [Google Scholar]
- Havránková, E.; Csöllei, J.; Pazdera, P. New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study. Molecules 2019, 24, 3586. [Google Scholar] [CrossRef] [PubMed]
- Havránková, E.; Csöllei, J.; Vullo, D.; Garaj, V.; Pazdera, P.; Supuran, C.T. Novel sulfonamide incorporating piperazine, aminoalcohol and 1,3,5-triazine structural motifs with carbonic anhydrase I, II and IX inhibitory action. Bioorg. Chem. 2018, 77, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Mut-Salud, N.; Álvarez, P.J.; Garrido, J.M.; Carrasco, E.; Aránega, A.; Rodríguez-Serrano, F. Antioxidant Intake and Antitumor Therapy: Toward Nutritional Recommendations for Optimal Results. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havránková, E.; Čalkovská, N.; Padrtová, T.; Csöllei, J.; Opatřilová, R.; Pazdera, P. New 1,3,5-Triazine Derivatives Incorporating Aminobenzenesulfonamide, Aminoalcohol, Piperazine, Chalcone or Stilbene Structural Motifs and Evaluation of Their Antioxidative Activity. Proceedings 2019, 41, 17. https://doi.org/10.3390/ecsoc-23-06598
Havránková E, Čalkovská N, Padrtová T, Csöllei J, Opatřilová R, Pazdera P. New 1,3,5-Triazine Derivatives Incorporating Aminobenzenesulfonamide, Aminoalcohol, Piperazine, Chalcone or Stilbene Structural Motifs and Evaluation of Their Antioxidative Activity. Proceedings. 2019; 41(1):17. https://doi.org/10.3390/ecsoc-23-06598
Chicago/Turabian StyleHavránková, Eva, Nikola Čalkovská, Tereza Padrtová, Jozef Csöllei, Radka Opatřilová, and Pavel Pazdera. 2019. "New 1,3,5-Triazine Derivatives Incorporating Aminobenzenesulfonamide, Aminoalcohol, Piperazine, Chalcone or Stilbene Structural Motifs and Evaluation of Their Antioxidative Activity" Proceedings 41, no. 1: 17. https://doi.org/10.3390/ecsoc-23-06598
APA StyleHavránková, E., Čalkovská, N., Padrtová, T., Csöllei, J., Opatřilová, R., & Pazdera, P. (2019). New 1,3,5-Triazine Derivatives Incorporating Aminobenzenesulfonamide, Aminoalcohol, Piperazine, Chalcone or Stilbene Structural Motifs and Evaluation of Their Antioxidative Activity. Proceedings, 41(1), 17. https://doi.org/10.3390/ecsoc-23-06598





