Sulfonated Graphitic Carbon Nitride (Sg-C3N4): A Highly Efficient Heterogeneous Organo-Catalyst for Condensation Reactions †
Abstract
:1. Introduction
2. Experimental
2.1. General
2.2. Preparation of g-C3N4 Nanosheets
2.3. Preparation of Sg-C3N4
2.4. General Method for the Synthesis of Imidazole Derivatives
2.5. General Method for Synthesis of Quinoxaline Derivatives
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Kumari, J. Application of Heterocyclic Compounds in Everyday Life. J. Mod. Chem. Chem. Technol. 2018, 9, 1–7. [Google Scholar]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, its derivatives and applications: A state of the art review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Shalini, K.; Sharma, P.K.; Kumar, N. Imidazole and its biological activities: A review. Chem. Sin. 2010, 1, 36–47. [Google Scholar]
- Samanta, P.K.; Banerjee, R.; Richards, R.M.; Biswas, P. Mesoporous silica supported ytterbium as catalyst for synthesis of 1, 2-disubstituted benzimidazoles and 2-substituted benzimidazoles. Appl. Organomet. Chem. 2018, 32, e4507. [Google Scholar] [CrossRef]
- Atanasova-Stamova, S.Y.; Georgieva, S.F.; Georgieva, M.B. Reaction strategies for synthesis of imidazole derivatives: A review. Scr. Sci. Pharm. 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Sonyanaik, B.; Ashok, K.; Rambabu, S.; Ravi, D.; Kurumanna, A.; Madhu, P.; Sakram, B. Facile One Pot Multi-Component Solvent-Free Synthesis of 2,4,5-Trisubstituted Imidazoles Using “Green” and Expeditious Ionic Liquid Catalyst under Microwave Irradiation. Russ. J. Gen. Chem. 2018, 88, 537–540. [Google Scholar] [CrossRef]
- Philbrook, G.E.; Maxwell, M.A.; Taylor, R.E.; Totter, J.R. Some relationships between structure and chemiluminescence in triphenylimidazoles. Photochem. Photobiol. 1965, 4, 1175–1183. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Kumar, A.; Liu, W.; Songsong, C.; Lin, Y. Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as Corros. inhibitors for J55 steel in sweet corrosive environment. J. Alloys Compd. 2017, 712, 121–133. [Google Scholar] [CrossRef]
- Allahvirdinesbat, M.; Fozi, M.; Safa, K.D.; Alyari, M.; Panahi, P.N.; Niaei, A. Synthesis of silyl-protected terminal thioalkyne-substituted tetraaryl imidazoles: Utilization of Ag–Fe/ZSM-5 bimetallic nanooxides for cyclocondensation of polysubstituted imidazoles. Res. Chem. Intermed. 2017, 43, 2653–2668. [Google Scholar] [CrossRef]
- Teimouri, A.; Chermahini, A.N.; Salavati, H.; Ghorbanian, L. An efficient and one-pot synthesis of benzimidazoles, benzoxazoles, benzothiazoles and quinoxalines catalyzed via nano-solid acid catalysts. J. Mol. Catal. A Chem. 2013, 373, 38–45. [Google Scholar] [CrossRef]
- Daragahi SA, H.; Mohebat, R.; Mosslemin, M.H. Green and Eco-Friendly Synthesis of Quinoxalines by Brönsted Acidic Ionic Liquid Supported on Nano-SiO2 under Solvent-Free Conditions. Org. Prep. Proced. Int. 2018, 50, 301–313. [Google Scholar] [CrossRef]
- Shirini, F.; Akbari-Dadamahaleh, S.; Mohammad-Khah, A.; Aliakbar, A.R. Rice husk: A mild, efficient, green and recyclable catalyst for the synthesis of 12-Aryl-8,9,10,12-tetrahydro [a] xanthene-11-ones and quinoxaline derivatives. Comptes Rendus Chim. 2013, 16, 207–216. [Google Scholar] [CrossRef]
- Shamsi-Sani, M.; Shirini, F.; Abedini, M.; Seddighi, M. Synthesis of benzimidazole and quinoxaline derivatives using reusable sulfonated rice husk ash (RHA-SO3H) as a green and efficient solid acid catalyst. Res. Chem. Intermed. 2016, 42, 1091–1099. [Google Scholar] [CrossRef]
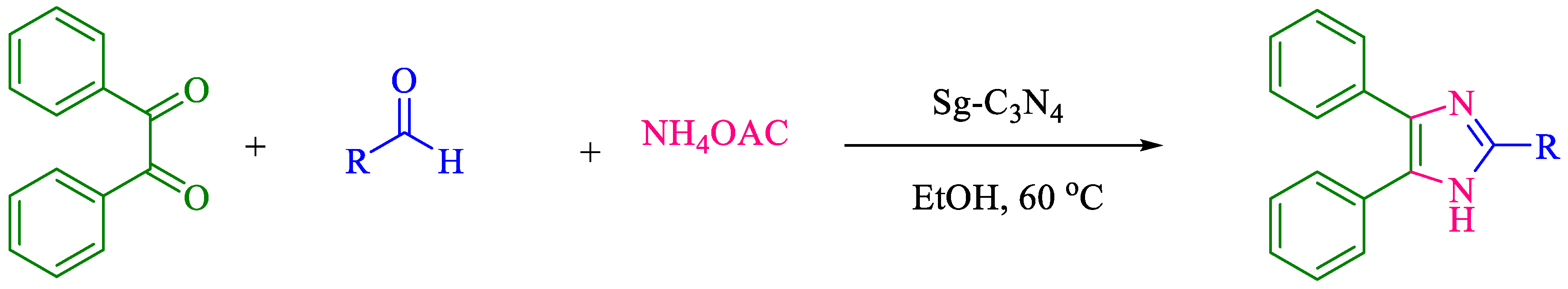

| Entry | Product | Time (min) | Yield (%) b | Mp °C (Ref.) |
|---|---|---|---|---|
| 1 |  | 10 | 94 | 276–280 [6] |
| 2 | 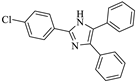 | 15 | 96 | 284–285 [7] |
| 3 |  | 15 | 95 | 241–242 [8] |
| 4 | 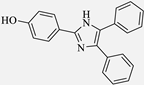 | 10 | 90 | 243–244 [9] |
| Entry | Dicarbonyl | Diamine | Product | Time (min) | Yield (%) b | Mp °C (Ref.) |
|---|---|---|---|---|---|---|
| 1 | 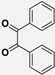 | 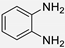 | 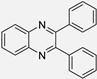 | 5 | 95 | 130–131 [10] |
| 2 | 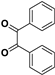 |  | 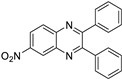 | 15 | 91 | 185–187 [11] |
| 3 | 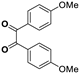 | 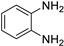 | 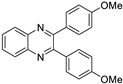 | 5 | 93 | 146–148 [12] |
| 4 | 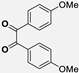 | 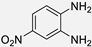 | 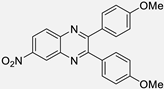 | 15 | 90 | 190–193 [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafuri, H.; Hanifehnejad, P.; Rezazadeh, Z.; Rashidizadeh, A. Sulfonated Graphitic Carbon Nitride (Sg-C3N4): A Highly Efficient Heterogeneous Organo-Catalyst for Condensation Reactions. Proceedings 2019, 41, 14. https://doi.org/10.3390/ecsoc-23-06611
Ghafuri H, Hanifehnejad P, Rezazadeh Z, Rashidizadeh A. Sulfonated Graphitic Carbon Nitride (Sg-C3N4): A Highly Efficient Heterogeneous Organo-Catalyst for Condensation Reactions. Proceedings. 2019; 41(1):14. https://doi.org/10.3390/ecsoc-23-06611
Chicago/Turabian StyleGhafuri, Hossein, Peyman Hanifehnejad, Zeynab Rezazadeh, and Afsaneh Rashidizadeh. 2019. "Sulfonated Graphitic Carbon Nitride (Sg-C3N4): A Highly Efficient Heterogeneous Organo-Catalyst for Condensation Reactions" Proceedings 41, no. 1: 14. https://doi.org/10.3390/ecsoc-23-06611
APA StyleGhafuri, H., Hanifehnejad, P., Rezazadeh, Z., & Rashidizadeh, A. (2019). Sulfonated Graphitic Carbon Nitride (Sg-C3N4): A Highly Efficient Heterogeneous Organo-Catalyst for Condensation Reactions. Proceedings, 41(1), 14. https://doi.org/10.3390/ecsoc-23-06611





