1. Introduction
Sewage sludge (SS) generated from wastewater treatment plants is produced in large quantities and, if not correctly managed, may pose harmful effects for the environment and public health [
1]. Conventional treatments like landfilling, incineration and application as fertilisers have been largely applied to manage SS, but the more stringent legislation that has been created discouraged the adoption of these solutions [
2]. Therefore, other treatment alternatives must be defined to value these residues and simultaneously to attenuate the negative impacts caused in the environment.
SS contains a high organic load giving them good calorific properties (11–23 MJ/kg db), thus with an interesting potential to be converted into useful energy [
1]. Gasification can be used for this purpose, since it reduces the consumption of fossil fuels and production of pollutant gases, and guarantees a continuous supply of energy for public use [
3]. Gasification of SS consists in a thermal conversion into a syngas with good fuel properties for energy production (1.3–5.6 MJ/m
3 [
1,
4]). The process occurs with lower amounts of oxygen when compared with combustion, therefore generating lower concentrations of SO
x, NO
x and polycyclic aromatic hydrocarbons. Furthermore, gasification may achieve better performances when compared with combustion [
3]. In the case of downdraft gasifiers, both waste and product gas flow downwards in the same direction, with the gas exiting at the bottom of the reactor. These gasifiers are known to create lower amounts of tars, requiring shorter start-up times and generating higher carbon-conversion efficiencies.
Because sewage sludge contains high moisture and ash contents that affects negatively the efficiency of the process, co-gasification with other residues has attracted a greater attention [
5]. Some works proposed the mixture with wood and acid hydrolysis residues from ethanol production, achieving interesting results for the syngas and process efficiency [
4,
5,
6,
7]. However, the literature is scarce regarding the analysis of co-gasification of SS and waste wood (WW) in downdraft gasifiers. This work aimed to evaluate the process behaviour and characterisation of products (gas and chars) resulting from the co-gasification of mixtures of SS and WW mainly from construction and demolition wastes (CDW), present in different proportions.
2. Materials and Methods
Dewatered SS was provided by a municipal wastewater treatment plant, and was submitted to a drying stage in a rotary drum dryer, followed by a solar drying exposure for around 5 days. Then, it was pelletized (Kahl 14-175, from Amandus Kahl GmbH & Co. KG, Reinbek, Germany) to obtain pellets with dimensions of 20 × 5 mm. WW (mainly from CDW) was supplied by a waste management company, and was sieved and screened to get chips with c.a. 40 mm, free of strange inert materials. Finally, three mixtures containing variable proportions of these wastes were prepared: M1 (100 wt.% WW), M2 (87.5 wt.% WW + 12.5 wt.% SS) and M3 (75 wt.% WW + 25 wt.% SS).
Raw wastes of SS and WW were analysed to determine contents of moisture, volatile matter and ash according to ASTM E949-88, E897-88 and E830-87, respectively; fixed carbon was calculated by difference. Ultimate analysis was carried out in a ThermoFisher Scientific Flash 2000 CHNS-O analyser (from Thermo Fisher Scientific, Waltham, USA), while higher heating value (HHV) was measured in a calorimetric bomb IKA C200 (from IKA-Werke GmbH & Co. KG, Staufen, Germany). Chlorine concentrations and mineral composition of ashes were determined by X-ray fluorescence analysis (Thermo Scientific Niton XL3t GOLDD+, from Thermo Fisher Scientific, Waltham, MA, USA).
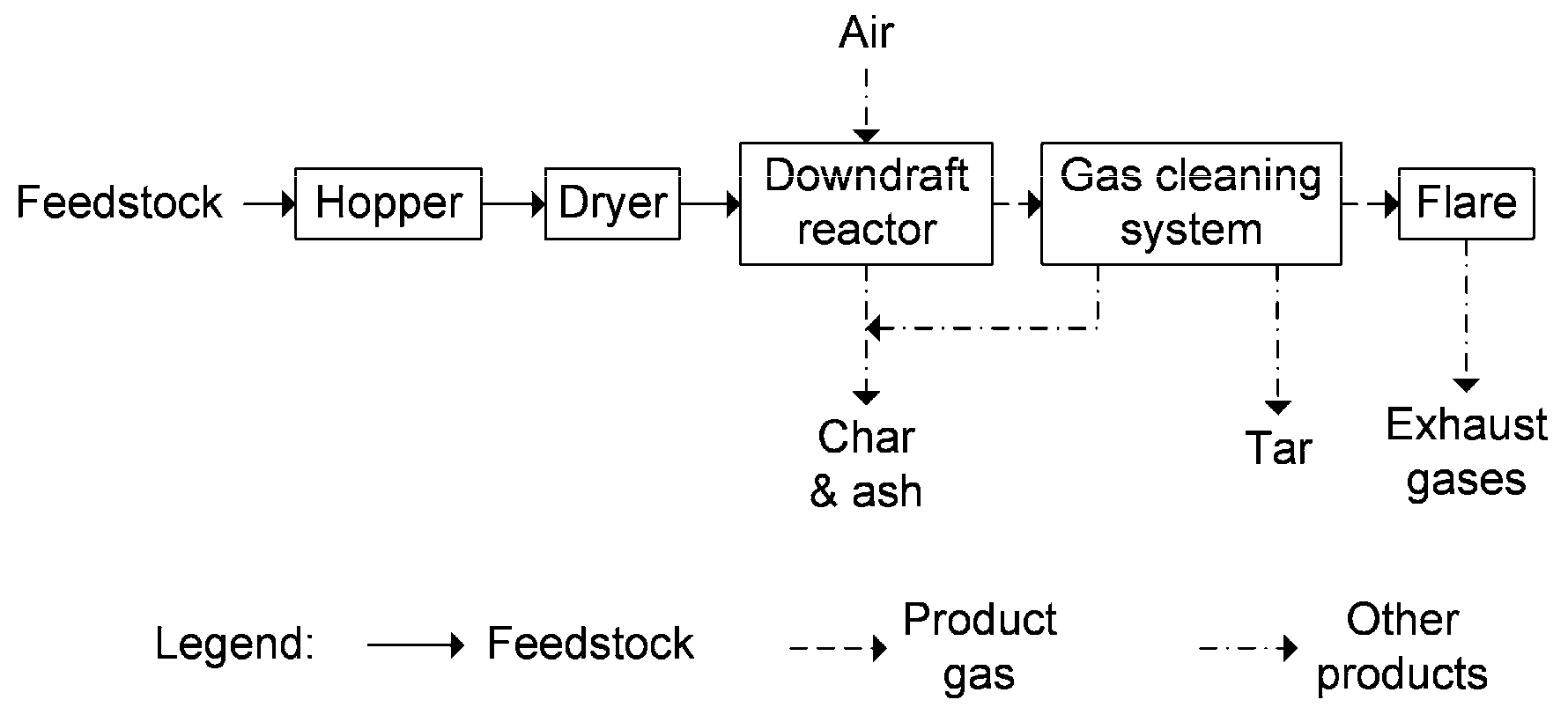
Gasification tests occurred in a downdraft gasifier (All Power Labs PP20, from All Power Labs, Berkeley, CA, USA), containing a reactor with 0.5 m height and 0.38 m diameter. A schematic of this gasifier is presented in
Figure 1.
The feedstock is stored in the hopper and, through the action of an auger mechanism, it is displaced to be dried and injected in the reactor. Inlet air is pre-heated by recirculating around the hot walls of the reactor. During the process, chars are periodically transferred to an auxiliary can, while the product gas moves to the cleaning system composed by a cyclone and a filter to retain particulates and tars. On the way between the cyclone and the filter, the gas is diverted to the drier in order to transfer heat to the feedstock. Finally, the gas goes to the flare to be burnt. When the temperature stabilised at 700–800 °C, two product gas samples were collected at the exit of the reactor into tedlar bags for further analysis. Chars and tars were also collected and quantified.
Gasification efficiency was evaluated through equivalence ratio (ER), yields of product gas, chars and tars, and cold-gas efficiency (CGE). ER was determined by the ratio of real and stoichiometric air flows. The real volumetric air flow (
Vair, m
3/s) and product gas yield (
Ygas, m
3/kg waste) were estimated through Equations (1) and (2), respectively [
8]:
with
poxid being the average vacuum pressure measured in the reactor (inH
2O),
mwaste and
mair the mass flows of waste and inlet air (kg/s),
xN waste and
xN2 gas the mass fractions of nitrogen present in the waste and in the gas, and
ρgas the mass density of the gas (kg/m
3).
Product gas samples were analysed to evaluate their composition by gas chromatography (Varian 450-GC, from Agilent, Santa Clara, CA, USA), equipped with two thermal conductivity detectors and two capillary columns (Ultimetal 1.5 × 0.002 m and 1 × 0.002 m). The LHV of gas samples and the corresponding density were calculated based on the proportions of each compound present in the gas, and on the individual calorific values and densities. Chars were analysed by their inorganic composition through an X-ray fluorescence analysis (Thermo Scientific Niton XL3t GOLDD+, from Thermo Fisher Scientific, Waltham, MA, USA).
3. Results and Discussion
Results for the analysis of raw wastes WW and SS are presented in
Table 1. SS contains a huge amount of moisture that has to be removed before gasification, since the process admits materials with a maximum content of 15 wt.% wb. This waste is also composed by a lower concentration of volatile matter and a significant amount of ash (69 and 23 wt.% db, respectively), when compared with WW. Therefore, high amounts of ash are expected after the tests, which would imply other post-treatments and a possible reduction in gasification efficiency. Nitrogen levels were relatively high due to the greater concentration of proteins [
1]. Although SS had lower H/C and O/C ratios (0.15 and 0.45, respectively) that are commonly observed in materials with high calorific values, the higher amount of ash limits its potential to produce energy; this fact may explain the reason why both wastes exhibit similar HHV’s (19 MJ/kg db). Corrosiveness of downstream equipment may not be a severe issue caused by the use of these wastes since the presence of chlorine is almost negligible (≤0.3 wt.% db). Regarding the mineral composition of ash, the presence of Fe and Ca was relevant in both residues (>23 wt.% db) indicating a strong trend for the production of agglomerations inside the reactor [
9]. This evidence is of particular concern in the situation of SS because of the high proportion of ash. Based on these results, the valorisation of SS through co-gasification with WW may be a viable solution to compensate the weaknesses of the former, once the volatile matter present in WW may increase the gas production, while ash production and agglomeration tendency may be reduced.
Table 2 exhibits the results for the gasification performance indicators. The addition of SS promoted an increase of product gas yield and CGE, as well as a considerable tar abatement that may prevent problems in downstream equipment. The rise of both gas yield and CGE was justified by the higher results observed for ER, which encouraged the formation of more gas at the exit. It appears that more air was needed for gasification in order to compensate the reduction of volatile matter of mixtures that included SS. CGE values were relatively higher for mixtures M2 and M3 (c.a. 85%) when compared with other works, which stated results around 70 % [
5]. This may suggest a possible overestimation of air flows calculated through correlation (1), and which contributed for the unexpected higher efficiencies. The incorporation of SS raised the production of chars especially in the situation of M2 (+45%). Although SS reduced tar generation, it also promoted the formation of chars as a by-product, and for which adequate treatments and valorisation pathways must be defined to divert their elimination to landfills. Mixture M3 conducted to the optimal results in terms of gasification performance, mainly due to the highest gas yield (3 m
3/kg waste), lowest tar formation (3.7 g/kg waste), and intermediary results for CGE (83.3%) and char yield (104 g/kg waste).
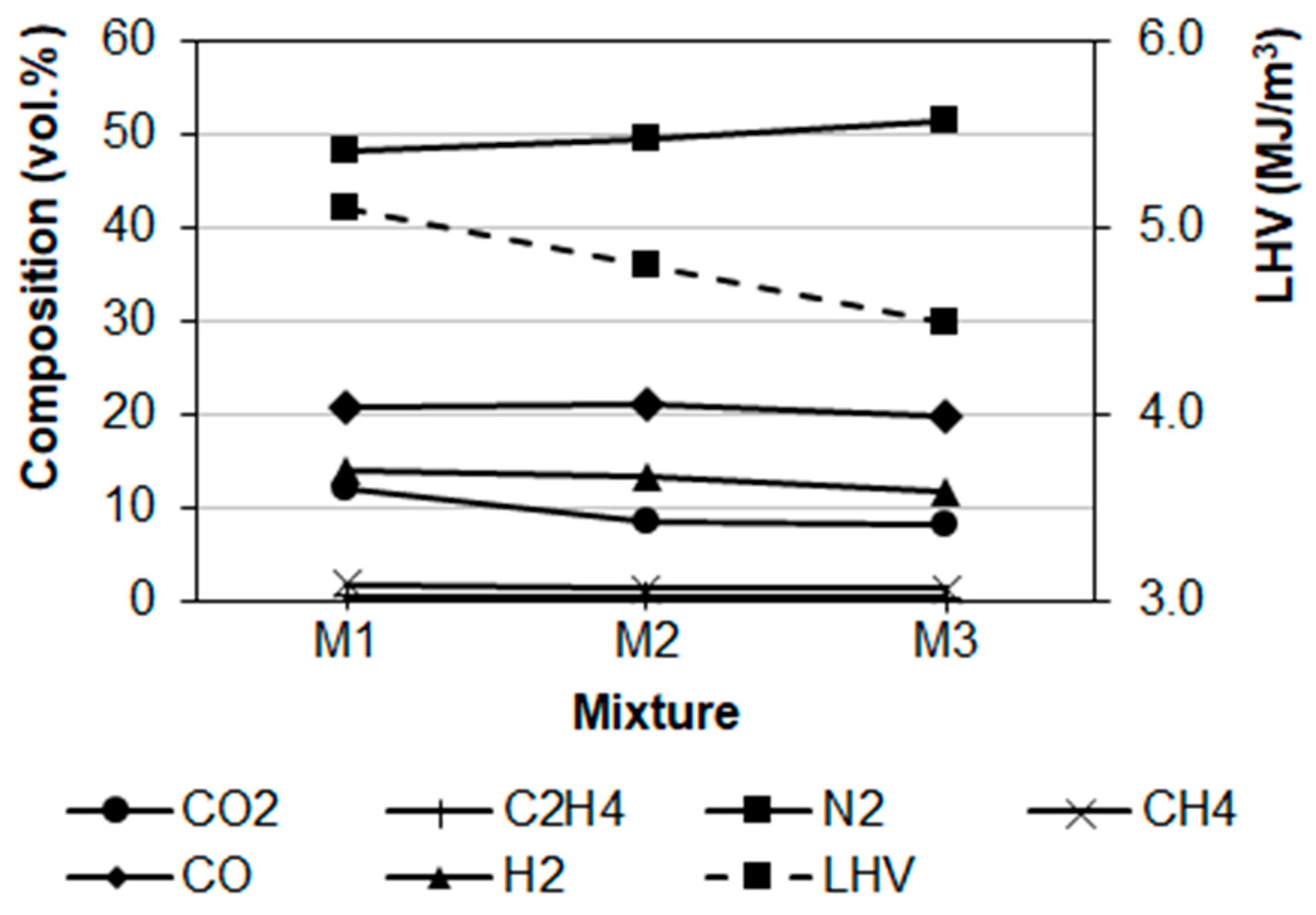
Evolution of composition and LHV of gas samples are represented in
Figure 2. The increase of SS proportion generated a reduction of H
2 and CH
4 concentrations, with a direct consequence in the diminishment of LHV. This is explained by three main reasons: (i) increase of the inorganic fraction introduced by SS, (ii) reduction of volatile matter content in the mixtures, and (iii) the decline of CH
4 affected negatively the steam methane reforming reaction that stimulates the formation of H
2 [
10]. An attenuation of CO
2 contents was verified, as well as in the case of CO when passing from mixture M2 to M3. These observations are in evident contradiction with the higher ER’s for mixtures containing SS; in fact, the increase of air flow introduced in the reactor would stimulate combustion reactions to produce more CO
2 [
6]. It seems that SS had a limiting effect in the reaction among carbon present in the feedstock and the oxygen from the air.
In order to investigate the properties and to establish valid pathways for the valorisation of chars,
Table 3 presents the levels of the main inorganic elements that were found. It was verified significant amounts of Ca and Fe oxides in all char samples (>6 wt.% db). The presence of Fe in high quantities justified the occurrence of stone agglomerations inside the reactor, with particular emphasis for M3. Greater concentrations of SS induced the formation of agglomerations of bigger dimensions and hardnesses, an observation that was also reported in [
5]. These agglomerations were thus responsible for blockage problems inside the reactor. Therefore, proportions of SS beyond 12.5 wt.% are not advisable to be incorporated in mixtures prepared for downdraft gasification.
Generally speaking, heavy metal concentrations were greater when SS was introduced in the mixtures. According to the Portuguese legislation (decree-law 103/2015, of June 15th), chars are not appropriate for valorisation as soil fertilisers due to the higher amounts of heavy metals that were analysed (with the exception of Hg), and which exceeded the legal limits. However, the presence of high concentrations of Ca, Fe and K allows their reuse as catalysts in a gasification process, with the aim of enhancing the decomposition of hydrocarbons and tar abatement [
11,
12].
Although the gasification performance was better when using the mixture M3, the formation of bigger agglomerations inside the reactor may eventually cause problems to the equipment and in the process operation. Therefore, the optimal proportions of WW and SS in the mixtures were those defined for the intermediate mixture M2 (87.5 wt.% WW + 12.5 wt.% SS), due to the reduced agglomerations inside the reactor, good results for LHV and yield of product gas (4.8 MJ/m3 and 2.9 m3/kg waste, respectively), lower tar formation (4.7 g/kg waste), and the highest CGE (86%).
4. Conclusions
In this work, co-gasification experiments by mixing WW and SS were performed in a downdraft reactor, aiming to investigate process performance and the properties of product gas and chars that were obtained. Inclusion of SS improved the CGE and product gas yield, and reduced the formation of tars that would induce operational and efficiency problems. Calorific properties of the product gas were not significantly affected when compared with the gas from the mixture with 100 wt.% WW. However, an increase of char production was observed, promoting the formation of agglomerations inside the reactor that caused blockage problems. These chars can be valorised as catalysts in gasification due to the high concentrations of Fe, Ca and K. The mixture that generated the optimal results was the one that incorporated 87.5 wt.% WW and 12.5 wt.% SS.
Co-gasification of WW and SS may be a valid alternative for the energetic valorisation of these residues, allowing to replace other conventional treatments like incineration and landfilling with harmful effects for the environment, and contributing for a better environmental sustainability.