Reduction of Total Phenols, Total Phosphorus and Turbidity by Uncatalytic Oxidation Processes in Cheese Whey Wastewater †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cheese Whey Wastewater Collection
2.3. Methods
2.4. Experimental Procedure
3. Results and Discussion
3.1. Cheese Whey Wastewater Characterization
3.2. Chemical Oxidation with Hydrogen Peroxide Alone
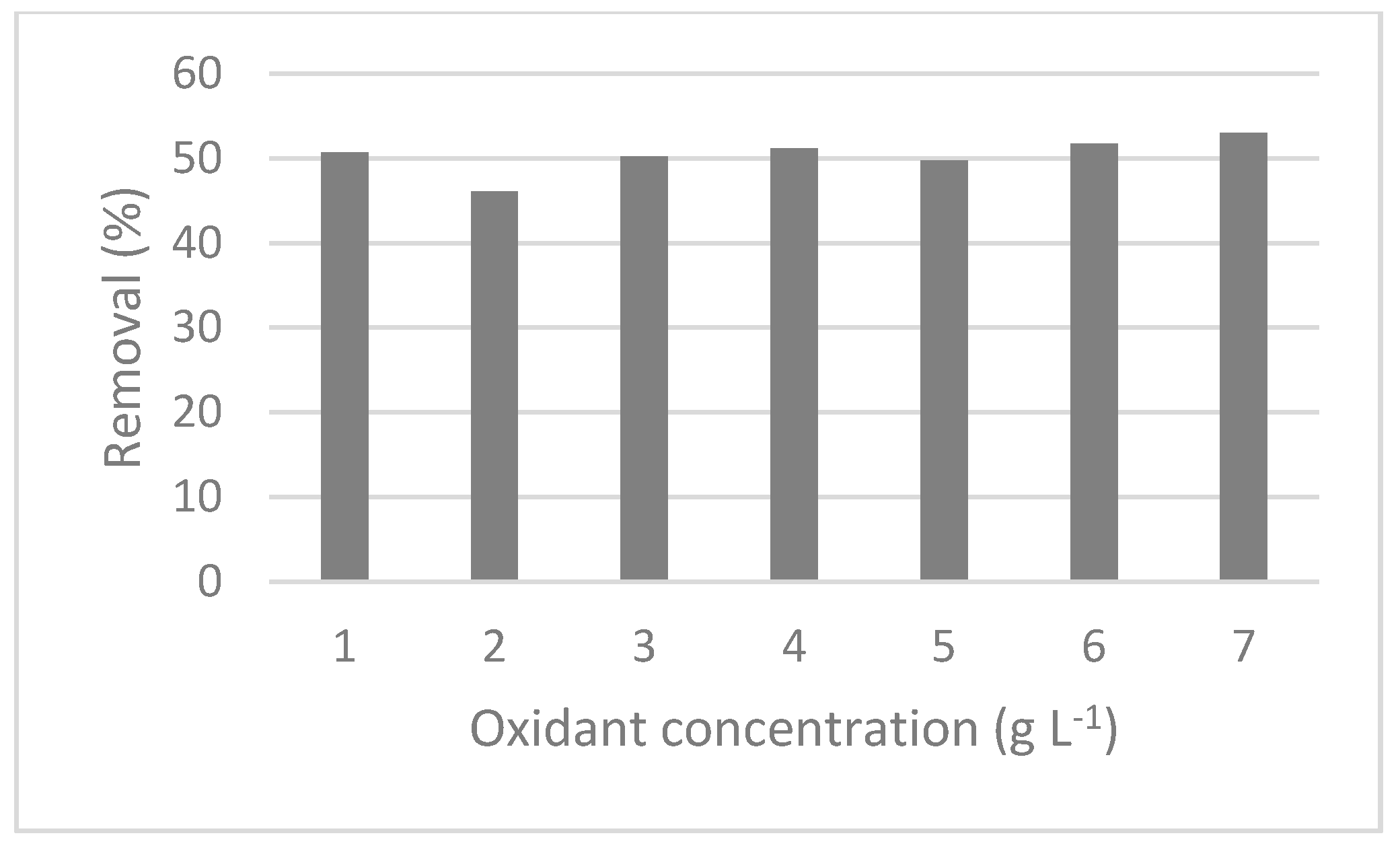
3.2.1. Total Phenols Removal
3.2.2. Total Phosphorus and COD Removal
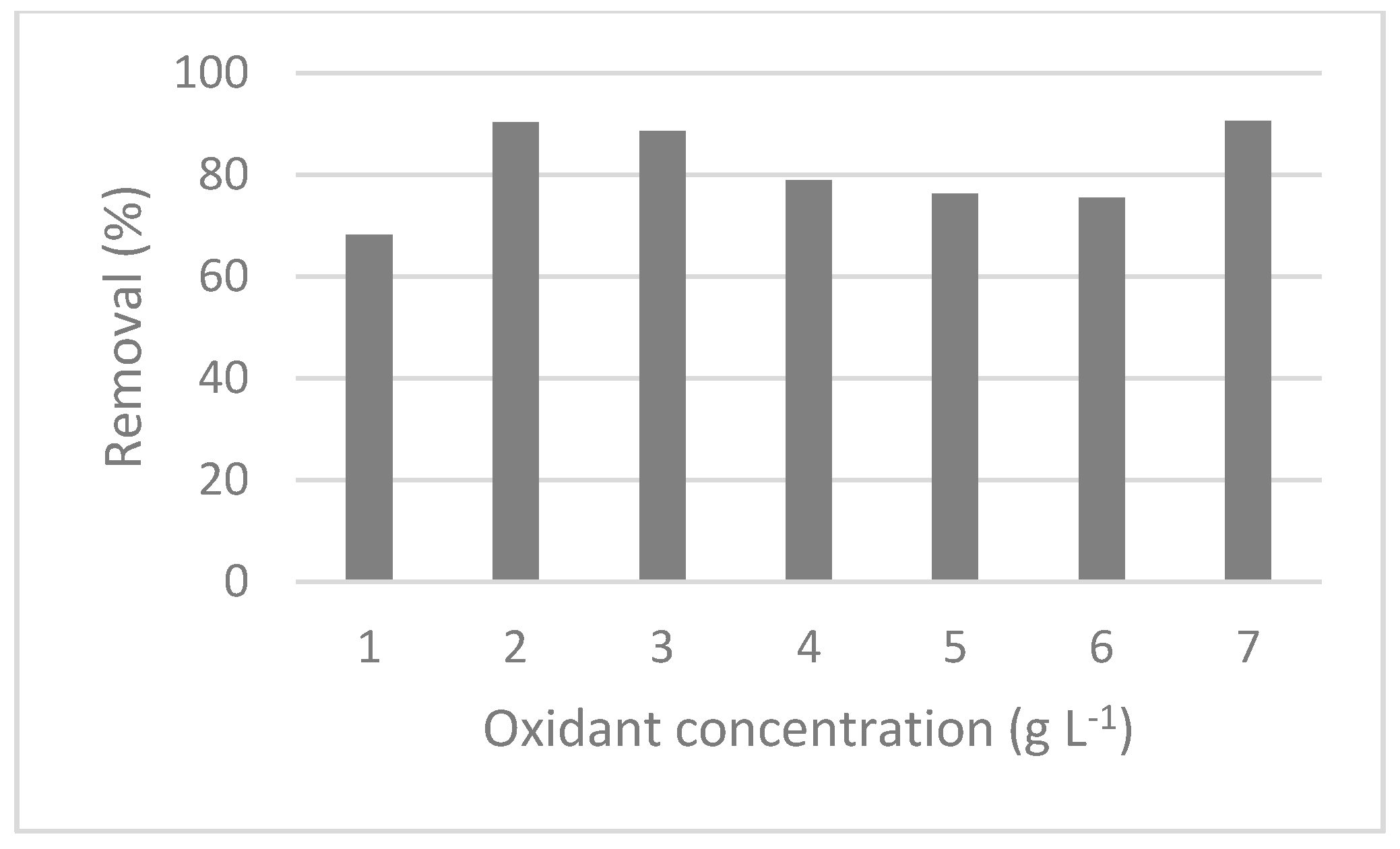
3.2.3. Turbidity Removal
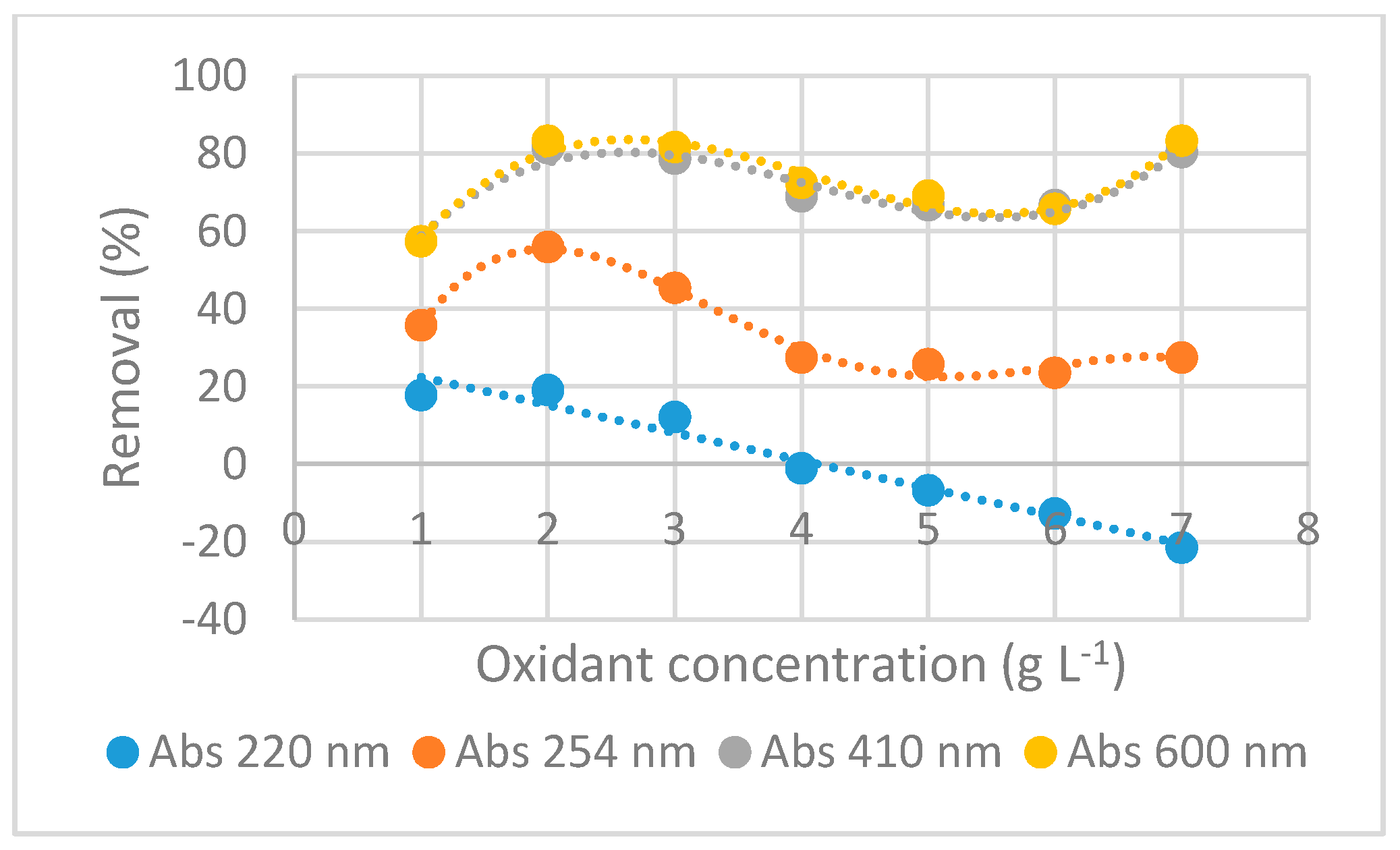
3.2.4. Characteristic Absorbances Removal
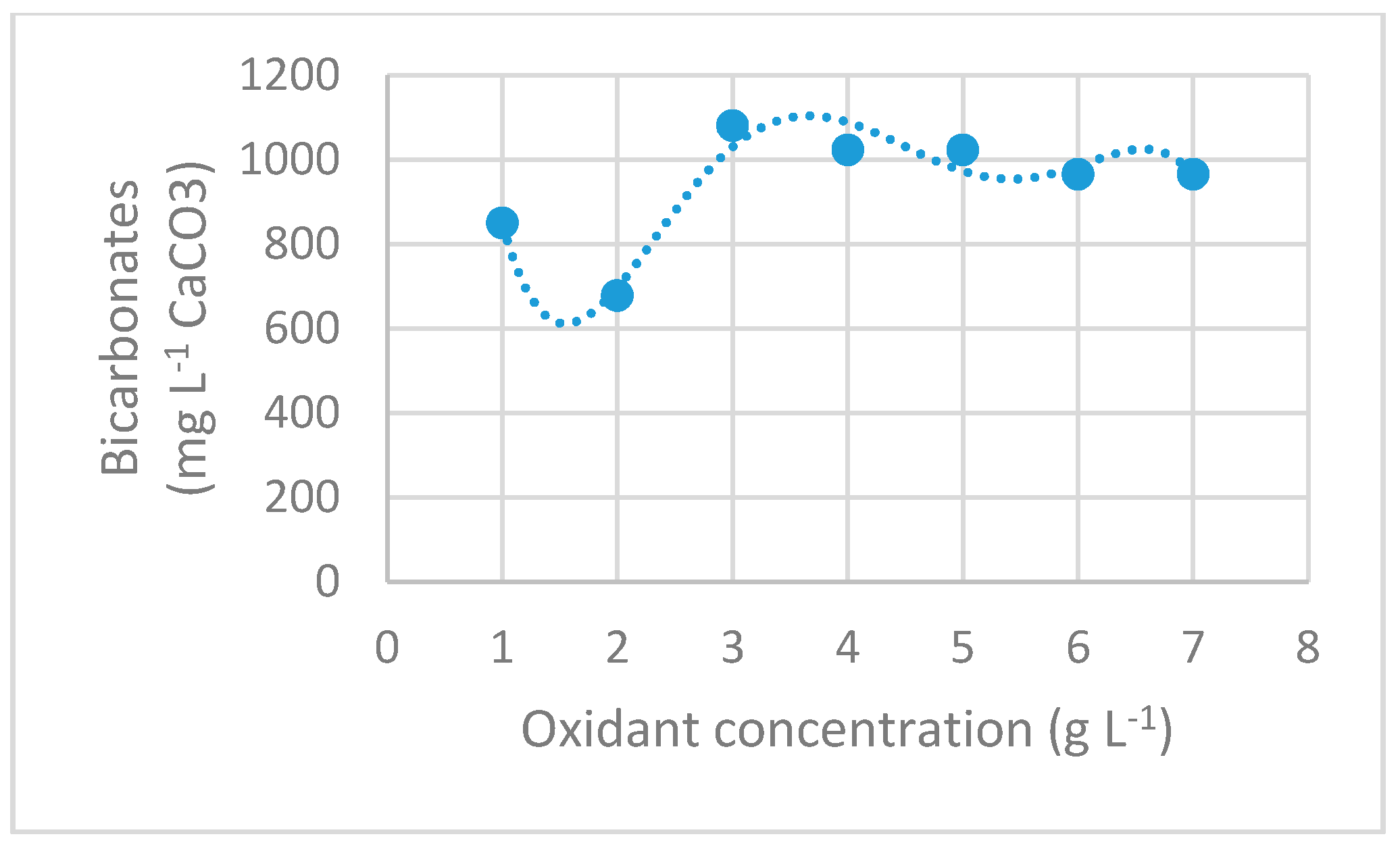
3.2.5. Bicarbonates Concentration
3.2.6. Calcium and Magnesium Concentration
3.2.7. pH and Conductivity
4. Conclusions
Acknowledgment
 |
References
- Dereli, R.K.; van der Zee, F.P.; Ozturk, I.; van Lier, J.B. Treatment of cheese whey by a cross-flow anaerobic membrane bioreactor: Biological and filtration performance. Environ. Res. 2019, 168, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.Y.; Mourti, C.; Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Effect of hydraulic retention time, temperature, and organic load on a horizontal subsurface flow constructed wetland treating cheese whey wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 726–732. [Google Scholar] [CrossRef]
- Tehrani, N.S.; Najafpour, G.D.; Rahimnejad, M.; Attar, H. Performance of upflow anaerobic sludge fixed film bioreactor for the treatment of high organic load and biogas production of cheese whey wastewater. Chem. Ind. Chem. Eng. Q. 2015, 21, 229–237. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.R.; Encina, P.A.G.; Fdz-Polanco, F. Anaerobic treatment of cheese-production wastewater using a UASB reactor. Bioresour. Technol. 1991, 37, 271–276. [Google Scholar] [CrossRef]
- Kalyuzhnyi, S.V.; Martinez, E.P.; Martinez, J.R. Anaerobic treatment of high-strength cheese-whey wastewaters in laboratory and pilot UASB-reactors. Bioresour. Technol. 1997, 60, 59–65. [Google Scholar] [CrossRef]
- Gavala, H.N.; Kopsinis, H.; Skiadas, I.V.; Stamatelatou, K.; Lyberatos, G. Treatment of dairy wastewater using an upfow anaerobic sludge blanket reactor. J. Agric. Eng. Res. 1999, 73, 59–63. [Google Scholar] [CrossRef]
- Fang, H.H.P. Treatment of wastewater from a whey processing plant using activated sludge and anaerobic processes. J. Dairy Sci. 1991, 74, 2015–2019. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Y.; Hwang, S. Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: Partial acidification and methanation. Water Res. 2003, 37, 2467–2477. [Google Scholar] [CrossRef]
- Rodgers, M.; Zhan, X.M.; Dolan, B. Mixing characteristics and whey wastewater treatment of a novel moving anaerobic biofilm reactor. J. Environ. Sci. Health 2004, 39, 2183–2193. [Google Scholar] [CrossRef]
- Rivas, J.; Prazeres, A.R.; Carvalho, F.; Beltrán, F. Treatment of cheese whey wastewater: Combined coagulation–flocculation and aerobic biodegradation. J. Agric. Food Chem. 2010, 58, 7871–7877. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Rivas, J.; Paulo, U.; Ruas, F.; Carvalho, F. Sustainable treatment of different high-strength cheese whey wastewaters: An innovative approach for atmospheric CO2 mitigation and fertilizer production. Environ. Sci. Pollut. Res. 2016, 23, 13062–13075. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.; Prazeres, A.R.; Carvalho, F. Aerobic biodegradation of precoagulated cheese whey wastewater. J. Agric. Food Chem. 2011, 59, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.; Carvalho, F.; Rivas, J. Cheese Whey Wastewater Treatment by Acidic Precipitation. In Proceedings of the XIV World Water Congress, International Water Resources Association and Water and Energy Resources Secretariat of Pernambuco State, Porto de Galinhas-PE, Brazil, 25–29 September, 2011. [Google Scholar]
- Prazeres, A.R.; Rivas, J.; Almeida, M.A.; Patanita, M.; Dôres, J.; Carvalho, F. Agricultural reuse of cheese whey wastewater treated by NaOH precipitation for tomato production under several saline conditions and sludge management. Agric. Water Manag. 2016, 167, 62–74. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J.; Patanita, M.; Dôres, J. Growth and development of tomato plants Lycopersicon Esculentum Mill. under different saline conditions by fertirrigation with pretreated cheese whey wastewater. Water Sci. Technol. 2013, 67, 2033–2041. [Google Scholar] [CrossRef]
- Martins, R.C.; Quinta-Ferreira, R.M. Final remediation of post-biological treated milk whey wastewater by ozone. Int. J. Chem. React. Eng. 2010, 8, 1542–6580. [Google Scholar] [CrossRef]
- Martins, R.C.; Rossi, A.F.; Castro-Silva, S.; Quinta-Ferreira, R.M. Fenton’s process for post-biologically treated cheese production wastewaters final remediation. Toxicity assessment. Int. J. Chem. React. Eng. 2010, 8, 1542–6580. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA-AWWA-WEF: Washington, DC, USA, 1998. [Google Scholar]
- Megharaj, M.; Avudainayagam, S.; Naidu, R. Toxicity of hexavalent chromium and its redution by bacteria isolated from soil contaminated with tannery waste. Curr. Microbiol. 2003, 47, 51–54. [Google Scholar] [CrossRef]
- Świetlik, J.; Dąbrowska, A.; Raczyk-Stanisławiak, U.; Nawrocki, J. Reactivity of natural organic matter fractions with chlorine dioxide and ozone. Water Res. 2004, 38, 547–558. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.; Carvalho, F.; Acedo, B.; Gimeno, O. Stabilized leachates: Sequencial coagulation-flocculation + chemical oxidation process. J. Hazard. Mater. 2004, 116, 95–102. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.; Carvalho, F.; Gimeno, O.; Frades, J. Study of Different Integrated Physical-Chemical + Adsorption Processes for Landfill Leachate Remediation. Ind. Eng. Chem. Res. 2005, 44, 2871–2878. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Raczyk-Stanisławiak, U.; Swietlik, J.; Nawrocki, J. Catalytic ozonation of natural organic matter on alumina. Appl. Catal. B 2006, 62, 345–358. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Álvarez-Uriarte, J.I.; González-Velasco, J.R. Enhanced coagulation under changing alkalinity-hardness conditions and its implications on trihalomethane precursors removal and relationship with UV absorbance. Sep. Purif. Technol. 2007, 55, 368–380. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 1994. [Google Scholar]
- Metcalf; Eddy. Wastewater Engineering, Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Madeira, L.; Fernandes, F.; Almeida, A.; Carvalho, F.; Jerónimo, E.; Prazeres, A.R. Aplicação de Oxidação Química para o Tratamento de Águas Residuais de Matadouro. In Proceedings of the 14° Congresso da Água: Gestão dos Recursos Hídricos Novos Desafios, Évora, Portugal, 7–9 March 2018. [Google Scholar]
- Ksibi, M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Zouari, N. Decolorization of olive oil mill effluent by physical and chemical treatment prior to anaerobic digestion. J. Chem. Technol. Biotechnol. 1998, 73, 297–303. [Google Scholar] [CrossRef]

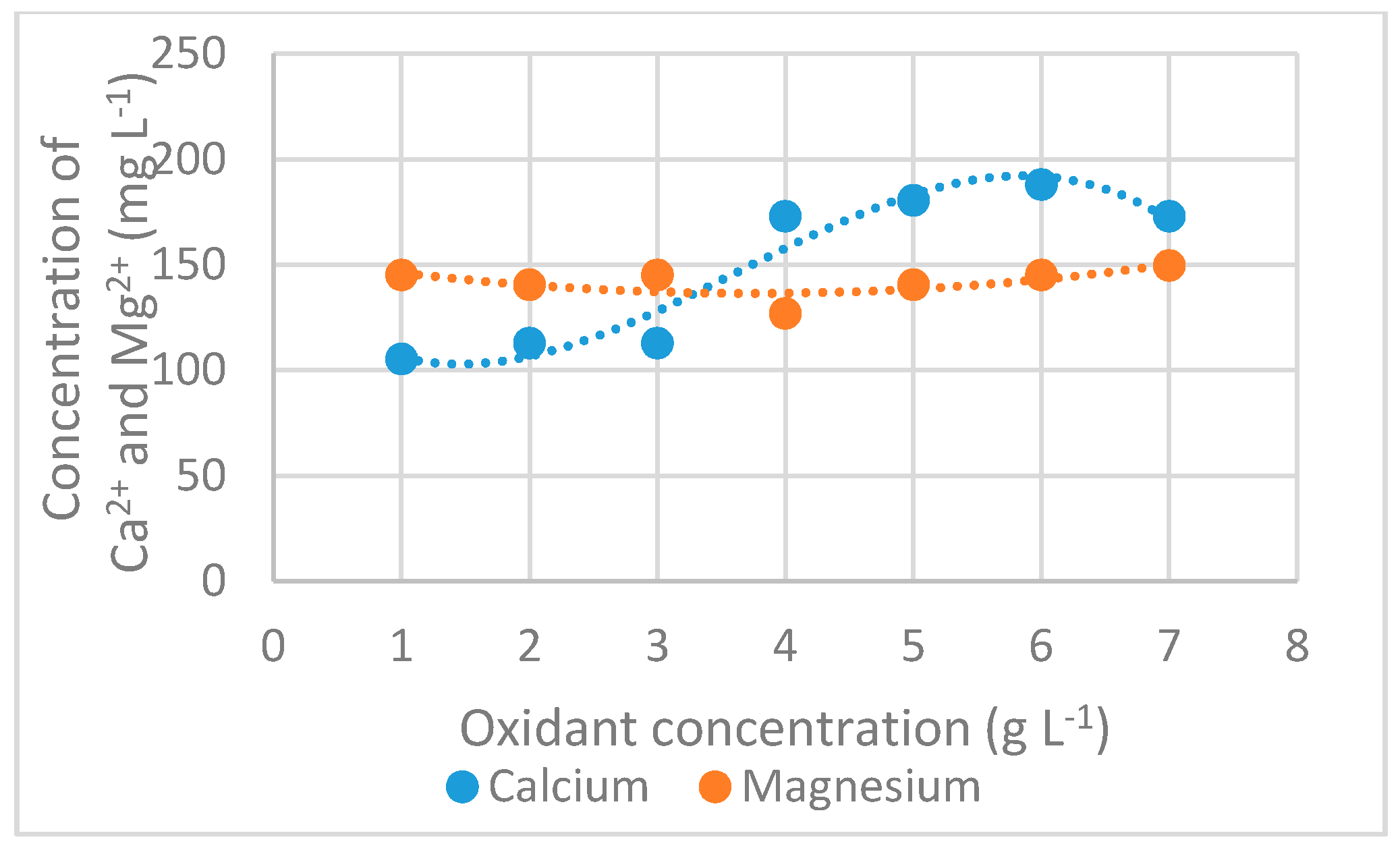
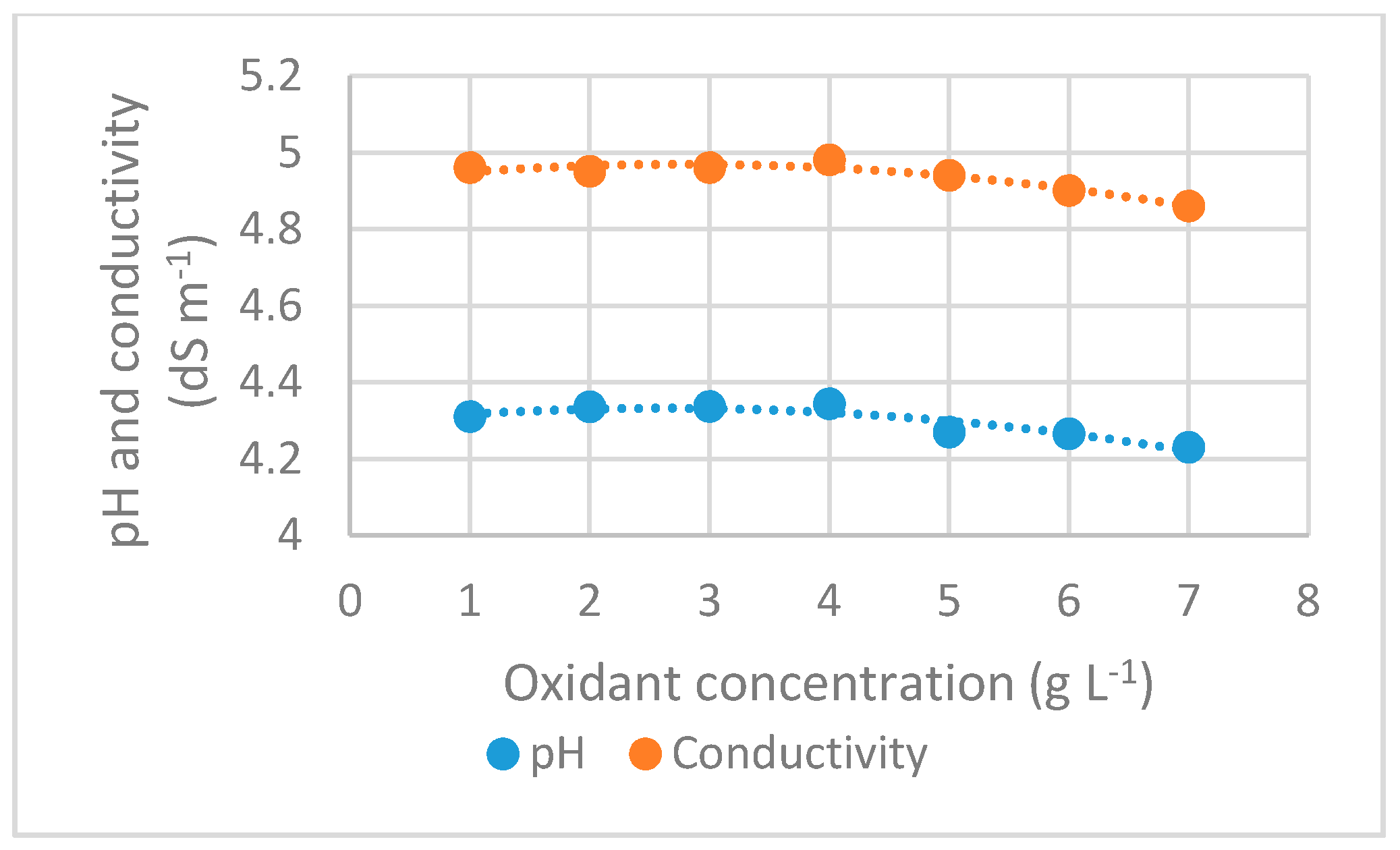
| Parameter | Units | Interval |
|---|---|---|
| pH | Sorensen scale | 4.162–4.675 |
| Conductivity | dS m−1 | 4.86–5.54 |
| Absorbance at 220 nm | (dilution 1:25) | 0.599–0.700 |
| Absorbance at 254 nm | (dilution 1:25) | 0.157–0.196 |
| Absorbance at 410 nm | (dilution 1:25) | 0.083–0.101 |
| Absorbance at 600 nm | (dilution 1:25) | 0.053–0.071 |
| Total alkalinity | mg CaCO3 L−1 | 1155.4–1708.5 |
| Bicarbonates | mg CaCO3 L−1 | 1155.4–1708.5 |
| Total Hardness | mg CaCO3 L−1 | 934.6–1347.5 |
| Calcic Hardness | mg CaCO3 L−1 | 487.9–675.6 |
| Magnesium Hardness | mg CaCO3 L−1 | 296.5–709.4 |
| Calcium | mg L−1 | 195.6–270.8 |
| Magnesium | mg L−1 | 72.1–172.4 |
| COD | mg L−1 | 4416.7–5250.0 |
| Turbidity | NTU | 536.49–659.73 |
| Total Phosphorus | mg L−1 | 1796.1–4894.1 |
| Total Phenols | mg equivalent of gallic acid L−1 | 65.00–82.86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prazeres, A.R.; Luz, S.; Fernandes, F.; Jerónimo, E. Reduction of Total Phenols, Total Phosphorus and Turbidity by Uncatalytic Oxidation Processes in Cheese Whey Wastewater. Proceedings 2019, 38, 16. https://doi.org/10.3390/proceedings2019038016
Prazeres AR, Luz S, Fernandes F, Jerónimo E. Reduction of Total Phenols, Total Phosphorus and Turbidity by Uncatalytic Oxidation Processes in Cheese Whey Wastewater. Proceedings. 2019; 38(1):16. https://doi.org/10.3390/proceedings2019038016
Chicago/Turabian StylePrazeres, Ana R., Silvana Luz, Flávia Fernandes, and Eliana Jerónimo. 2019. "Reduction of Total Phenols, Total Phosphorus and Turbidity by Uncatalytic Oxidation Processes in Cheese Whey Wastewater" Proceedings 38, no. 1: 16. https://doi.org/10.3390/proceedings2019038016
APA StylePrazeres, A. R., Luz, S., Fernandes, F., & Jerónimo, E. (2019). Reduction of Total Phenols, Total Phosphorus and Turbidity by Uncatalytic Oxidation Processes in Cheese Whey Wastewater. Proceedings, 38(1), 16. https://doi.org/10.3390/proceedings2019038016




