Unusual Salivary Gland Tumor of the Palate: Clinical, Histological and Immunohistochemical Features †
1. Introduction
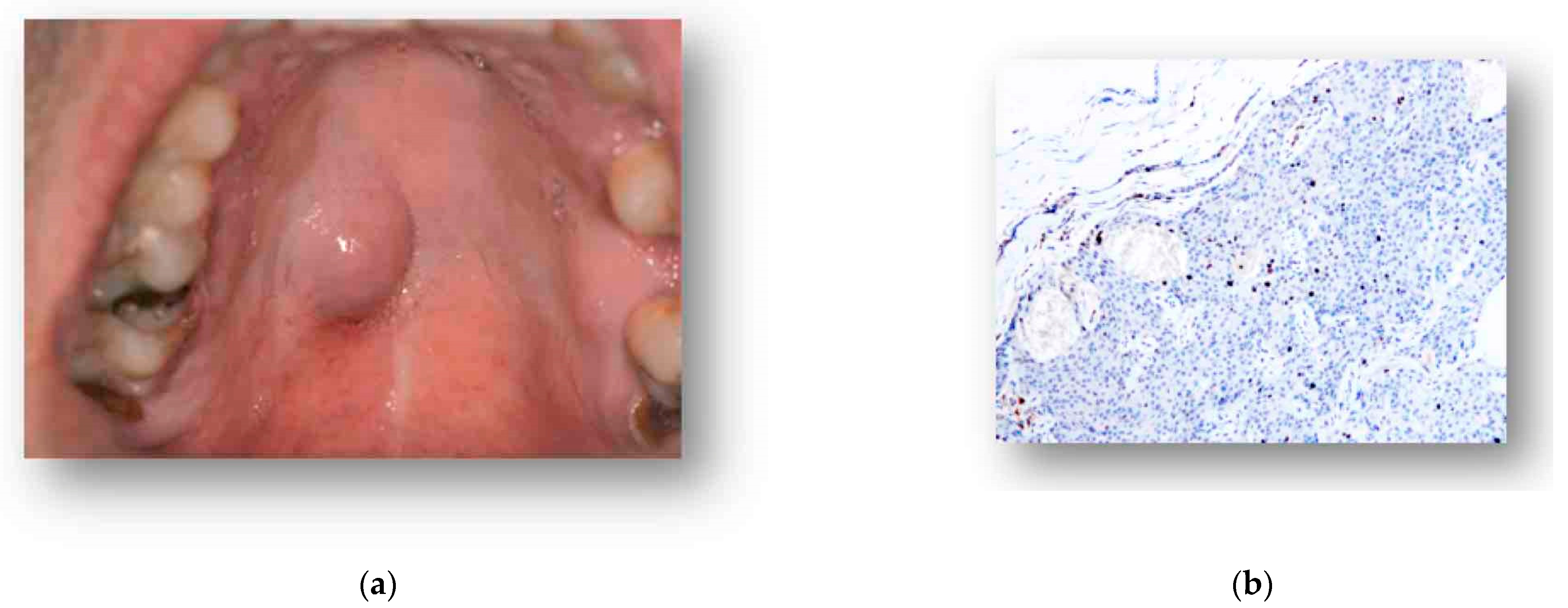
2. Case Presentation
3. Discussion
Conflicts of Interest
References
- IARC. WHO Classification of Tumors, 4th ed.; IARC press: Lyone, France, 2017; pp. 159–194. [Google Scholar]
- Carlson, E.R.; Schlieve, T. Salivary Gland Malignancies. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Galdirs, T.M.; Kappler, M.; Reich, W.; Eckert, A.W. Current aspects of salivary gland tumors—A systematic review of the literature. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2019, 8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzi, M.; Vella, F.D.; Sportelli, P.; Maiorano, E. Unusual Salivary Gland Tumor of the Palate: Clinical, Histological and Immunohistochemical Features. Proceedings 2019, 35, 7. https://doi.org/10.3390/proceedings2019035007
Petruzzi M, Vella FD, Sportelli P, Maiorano E. Unusual Salivary Gland Tumor of the Palate: Clinical, Histological and Immunohistochemical Features. Proceedings. 2019; 35(1):7. https://doi.org/10.3390/proceedings2019035007
Chicago/Turabian StylePetruzzi, Massimo, Fedora Della Vella, Pasquale Sportelli, and Eugenio Maiorano. 2019. "Unusual Salivary Gland Tumor of the Palate: Clinical, Histological and Immunohistochemical Features" Proceedings 35, no. 1: 7. https://doi.org/10.3390/proceedings2019035007
APA StylePetruzzi, M., Vella, F. D., Sportelli, P., & Maiorano, E. (2019). Unusual Salivary Gland Tumor of the Palate: Clinical, Histological and Immunohistochemical Features. Proceedings, 35(1), 7. https://doi.org/10.3390/proceedings2019035007




