1. Introduction
The e-textile is a combination of electrical systems in clothing and accessories. These electrical systems consume electrical energy that requires an electrical power supply and buffer modules [
1]. Such a system could benefit from a flexible energy storage device with high cycling stability, energy, and power density. A supercapacitor is an electrochemical device that can store more electrical energy than a conventional capacitor while having better power density and cycling stability than a secondary battery. It can be applied as an energy reservoir for existing devices in an e-textile system. At present, e-textile systems are typically powered by conventional secondary batteries that lack flexibility, are limited by their power density, and have poor cycling stability that requires frequent replacement [
2]. These limitations mean secondary batteries are not always compatible with e-textile designs. Therefore, new flexible and efficient energy storage devices like textile supercapacitors are of major interest as energy suppliers or buffers for smart fabric applications and e-textile approaches, but these options have not been fully developed.
Previously, Cheng et al. [
3] demonstrated a flexible supercapacitor with a nick-metal-framework and reduced graphene forming the fabric electrode. The flexible supercapacitor with a highly concentrated alkaline electrolyte demonstrated an area capacitance of 95 mF·cm
−2. Nie et al. [
4] reported a fiber-shaped flexible supercapacitor with a graphene oxide, poly(pyrrole), and poly (lactic acid) filament electrode. The flexible supercapacitor was characterized with phosphoric acid gel electrolyte and achieved a high areal capacitance of 158.8 mF·cm
−2. In both cases, the electrolyte contained hazardous materials and used expansive carbonized materials like graphene oxide.
A supercapacitor (electrical double layer type) is constructed using a two electrical double layer interfaces, with each having opposite polarity with respect to the electrolyte solution. Generally, a supercapacitor can be realized with two layers of textile electrode sandwiching a filter paper as a separator. Researchers have used hazardous substances in their supercapacitors, such as strong acid [
5] in the gel electrolyte or corrosive oxide material [
6] in the electrode, while these devices also consist of two layers of fabric electrode and a paper separator. The material and the configuration of supercapacitors require extra consideration when using packaging that reduces the potential for skin irritation and compresses/seals the multilayer device. Therefore, although such fabric supercapacitors achieve good electrochemical performances, the use of hazardous materials and a multilayer device structure mean they are not the best opinion for e-textiles.
This paper reports an approach for integrating the supercapacitor alongside e-textile electronics. The electrodes of a supercapacitor were fabricated with a spray coating process using inexpensive carbon powders. The separator was implemented in the polyester-cotton fabrics before using an electrodes spray coating process. The flexible textile supercapacitor was encapsulated and tested with an ionic liquid electrolyte in order to study their electrochemical performance.
2. Materials and Methods
The proposed supercapacitor was fabricated using a woven polyester-cotton fabric, solution-based processes, and inexpensive materials. The porous woven polyester-cotton was first screen-printed with a co-polymer solution which contained ethylene-vinyl acetate (EVA) and poly methyl methacrylate (PMMA), and was treated with a phase inversion process to form a porous membrane of 25 µm in the fabric. This co-polymer membrane acts as a separator of the supercapacitor and holds the electrolyte, prevents electrical short circuitry, but allows ions transfer between the top and bottom electrodes. The supercapacitor electrodes were spray coated onto different sides of the textile through a mask. The carbon solution for spray coating contains solvent, 15 wt% of polymer binder ethylene-vinyl acetate (EVA) and 5 wt% of carbon black. During the spray coating process, carbon mist adheres to the polyester-cotton fabrics uniformly, penetrates into the textile, and is stopped by the polymer membrane. After solvent evaporation, the carbon particles bonds to the textiles, forming a conductive and porous electrode. The supercapacitor cell was impregnated with a low hazardous gel and ionic conductive sulfoxide-based electrolyte solution, cured in an oven for 2 h at 60 °C, sandwiched between a pair of nickel coated copper films, and encapsulated by EVA polymer films using a heat press.
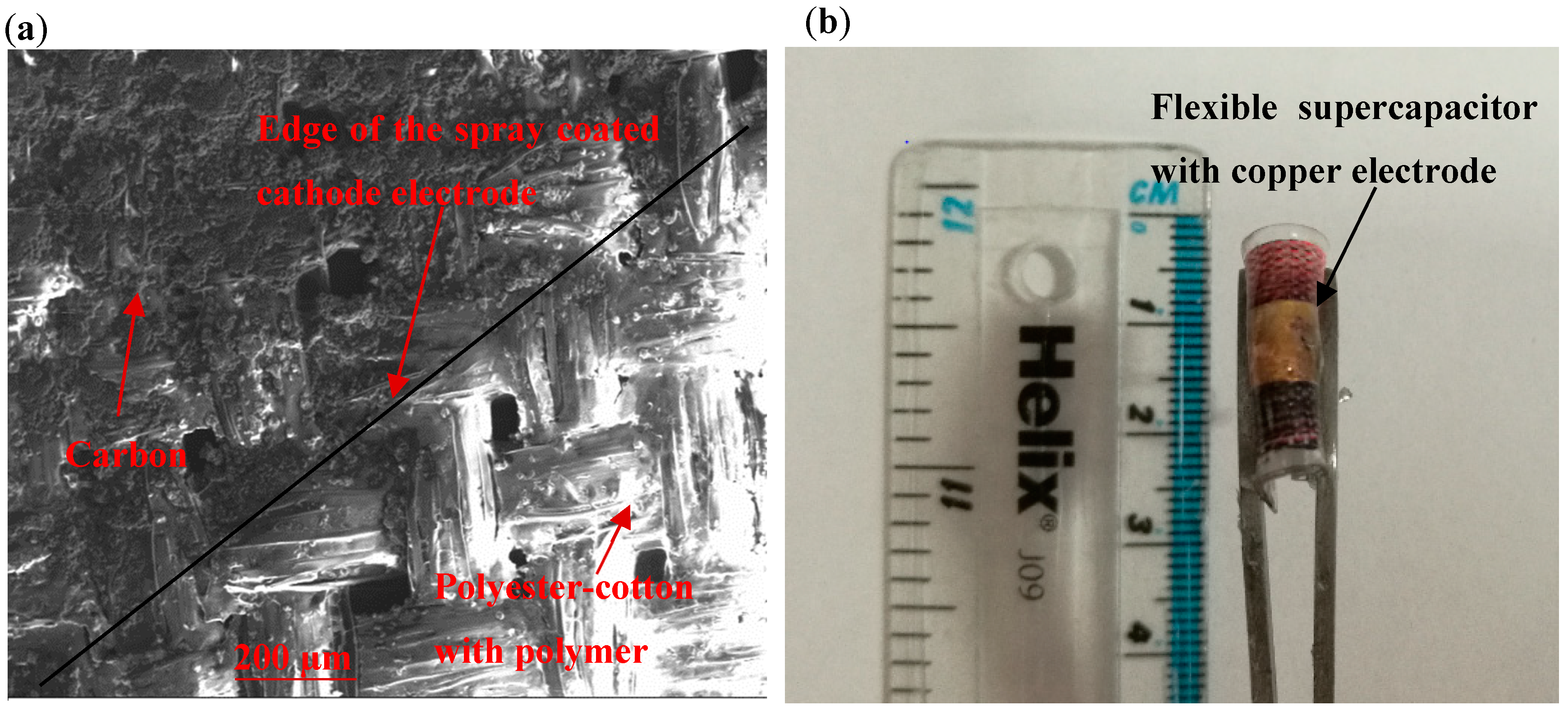
Figure 1a shows the Scanning electron microscope (SEM) picture of the spray coated carbon electrode on the porous membrane coated polyester-cotton fibers. The carbon particles adhered to the polyester-cotton yarns to form a conductive network. The flexible encapsulated supercapacitor (
Figure 1b) has a surface area of 0.785 cm
2 with thickness of about 300 μm, while each single piece of fabric electrode has a weight of 27.1 mg.cm
−2 before coating and carbon materials account for 13.4% of the final electrode weight.
3. Results
The solid-state fabric supercapacitor was tested using a VMP2 potentiostat/galvanostat (Biologic, France). The encapsulated supercapacitor was characterized by cyclic voltammetry (CV) at different scan rates and galvanostatic cycling (GC) at different scan voltage or current.
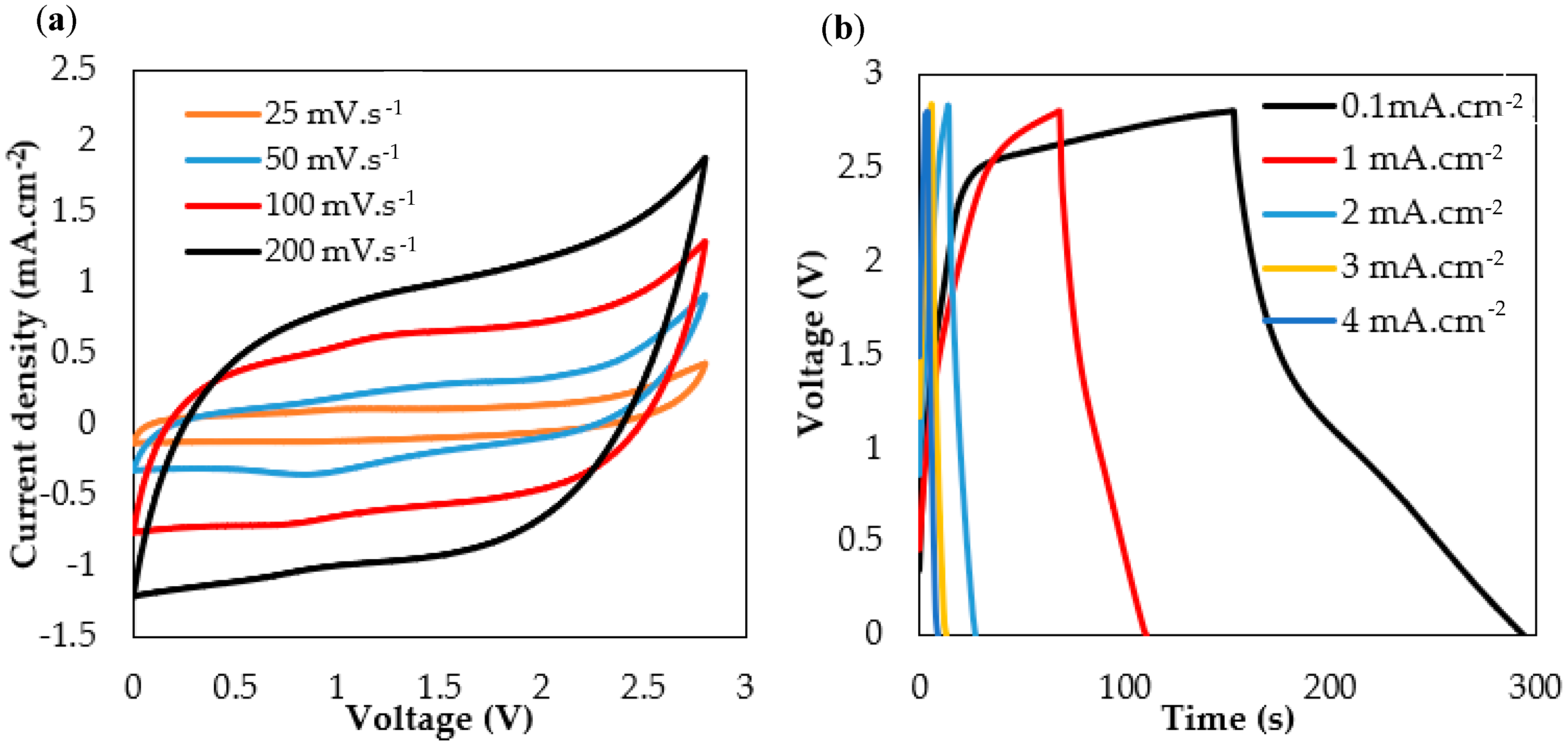
The cyclic voltammetry curve (CV) test of the flexible textile supercapacitor (
Figure 2a) shows that the device is electrochemically stable at the scan rates from 25 to 200 mV, and area capacitance values vary from about 24.5 to 11.7 mF·cm
−2 depending on the scan rate. The GC tests in
Figure 2b were at current densities from 0.1 to 4 mA cm
−2. At 1 mA cm
−2, the textile supercapacitor achieves an equivalent series resistance (ESR) of 354 Ω·cm
−2 specific area capacitances of 20.6 mF·cm
−2, but this declines with an increasing cycling current.
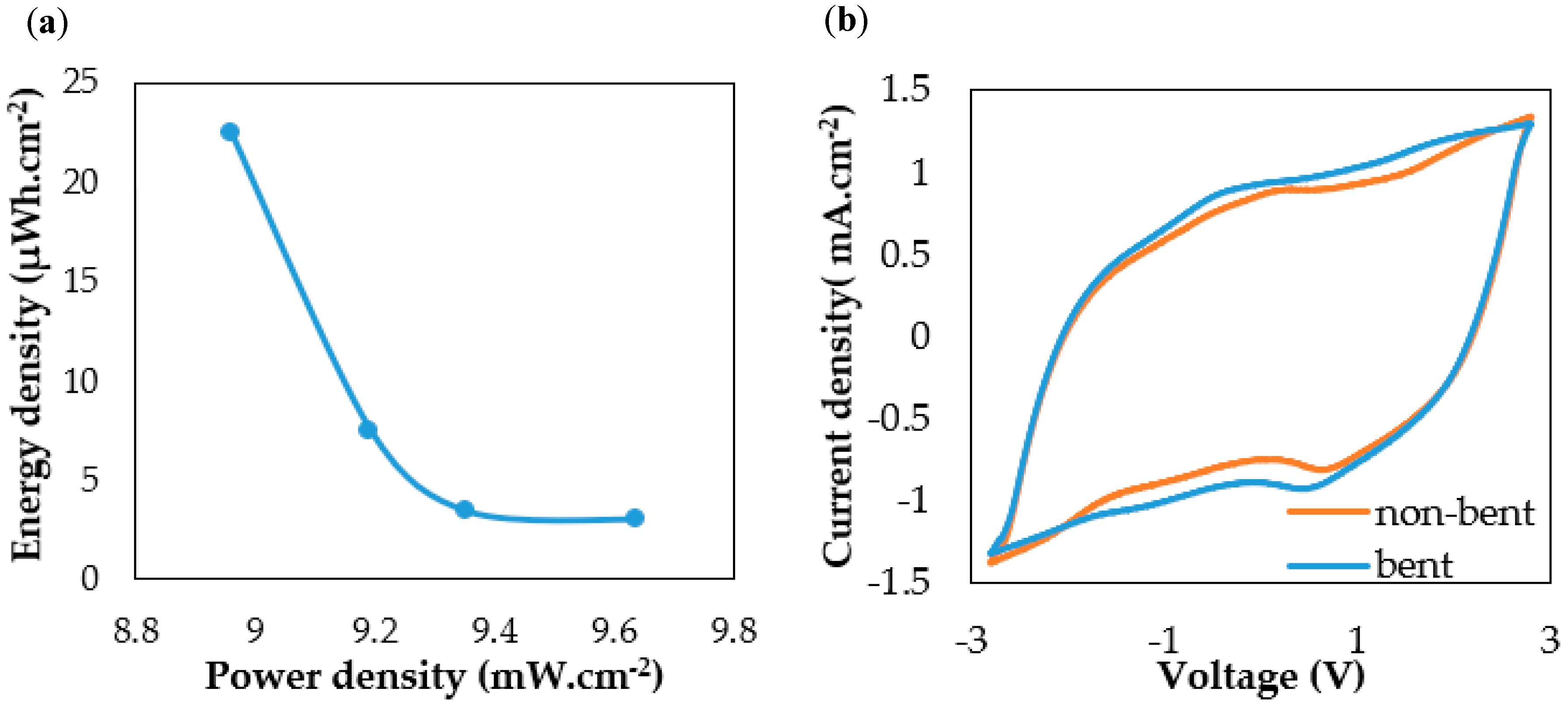
The Ragone plot in
Figure 3a was derived from GC tests between 1 and 4 mA·cm
−2; the area energy density deceased from 22 to 2.9 µWh·cm
−2 while its device power density increased from 8.9 to 9.6 mW·cm
−2. The encapsulated supercapacitors were repetitively bent 180 degrees 200 times.
Figure 3b shows the bending test result of the textile supercapacitor (non-bent and bent) between −2.8 V to 2.8 V. The shape and values of the CV result of the bent sample shows a small difference in comparison with the non-bent device’s CV result. This indicates that the textile supercapacitor is electrochemically stable against repeated bending. The high device bending stability indicates a flexible and durable polymer separator that resists electrical short circuiting under mechanical stress. The spray coating process and the use of EVA binder results in carbon particles being spread uniformly over the textile sample, but then stopped by the polymer separator. The use of gel and ionic conductive electrolyte with the vacuum impregnation process improved the wettability between the electrolyte and the electrode materials to form an efficient double layer interface during device testing.
4. Discussion and Conclusions
This paper presents an encapsulated flexible supercapacitor on a single piece of polyester-cotton. The supercapacitors fabricated in this work were electrochemically stable between +/−2.8 V and demonstrated an area specific capacitance of 20.6 mF·cm−2, a normalized ESR of 354 Ω·cm−2 and a good bending stability of a negligible difference CV result between the bent and non-bent devices. In comparison with other works, these results are not as good as for a formal multilayer non-encapsulated supercapacitor, however this proves that it is possible to implement an encapsulated supercapacitor in a single layer of polyester-cotton fabrics for e-textile systems. Future work will include optimizing the formulation and fabrication methods of the polymer membrane in the fabrics so more electrode material and the electrolyte can be loaded into the textile for better electrochemical performances. The final device can be applied in a wide range of t-textile systems.