A Convolutional Neural Network-Based Method for Human Movement Patterns Classification in Alzheimer’s Disease †
Abstract
:1. Introduction
2. Related Work
3. Method
3.1. Problem Description
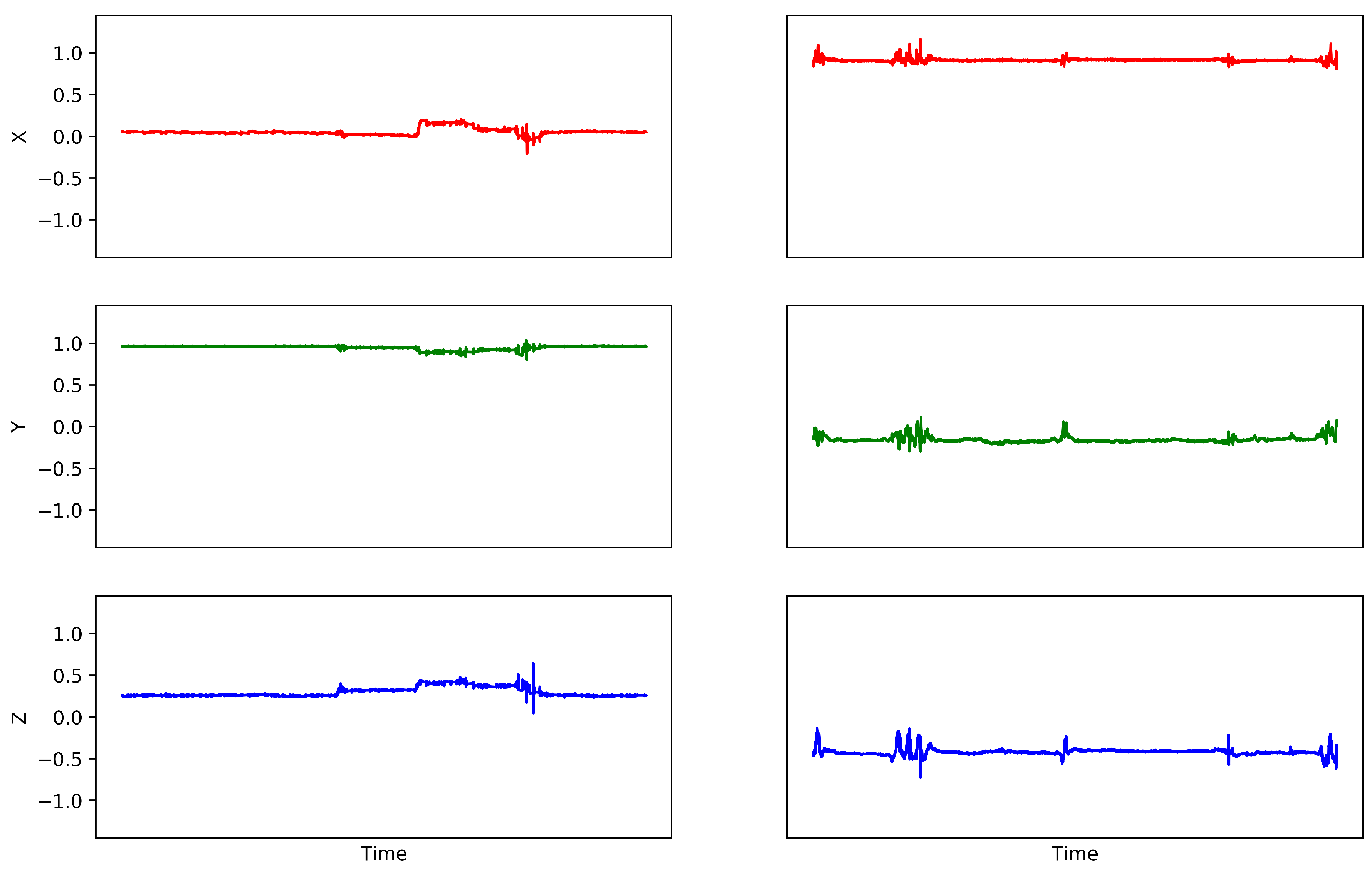
3.2. Data Preprocessing
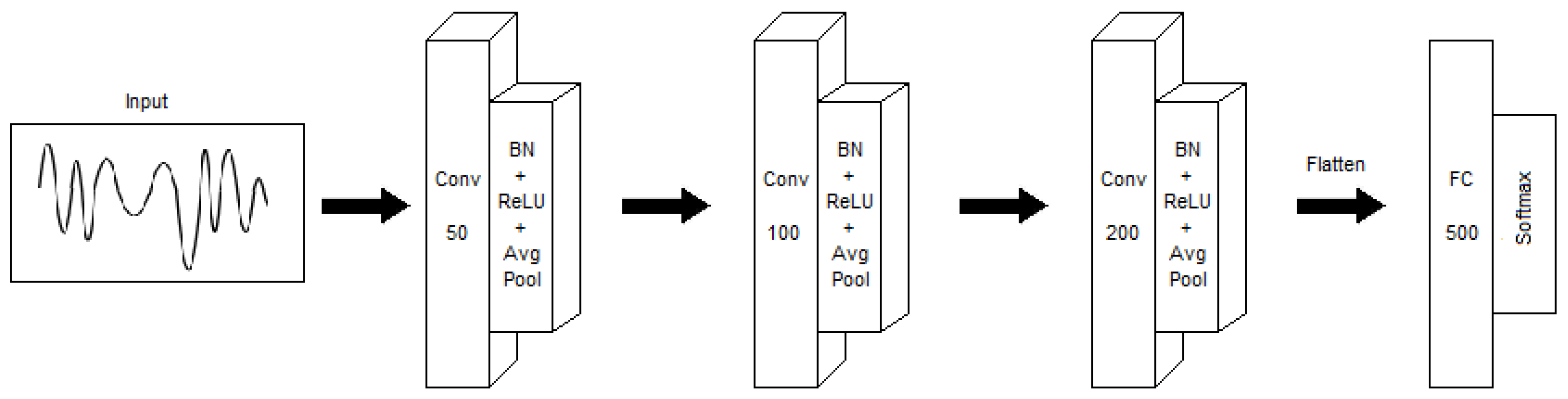
3.3. Convolutional Neural Network Classifier
4. Results
4.1. Data Description
4.2. Model Training
4.3. Model Evaluation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| CNN | Convolutional Neural Network |
| GDS | Global Deterioration Scale |
| MCI | Mild Cognitive Impairment |
| MLP | Multi-Layer Perceptron |
| RF | Random Forest |
| RT | Random Tree |
| SVM | Support-Vector Machine |
References
- Weiser, M.; Gold, R.; Brown, J.S. The Origins of Ubiquitous Computing Research at PARC in the Late 1980s. IBM Syst. J. 1999, 38, 693–696. [Google Scholar] [CrossRef]
- Deen, M.J. Information and Communications Technologies for Elderly Ubiquitous Healthcare in a Smart Home. Pers. Ubiquitous Comput. 2015, 19, 573–599. [Google Scholar] [CrossRef]
- R Varma, V.; Watts, A. Daily Physical Activity Patterns During the Early Stage of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2016, 55. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/wearable devices opportunity. Npj Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.; Wu, Y.; Prina, M. World Alzheimer Report 2015; Alzheimer’s Disease International: London, UK, 2015; pp. 1–92. [Google Scholar] [CrossRef]
- World Health Organization. Dementia: A Public Health Priority; World Health Organization: Geneva, Switzerland, 2012; pp. 1–4. ISBN 9789241564458.
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Leger, D.; Elbaz, M.; Dubois, A.; Rio, S.; Mezghiche, H.; Carita, P.; Stemmelin, J.; Strauss, M. Alzheimer’s Disease Severity is Not Significantly Associated with Short Sleep: Survey by Actigraphy on 208 Mild and Moderate Alzheimer’s Disease Patients. J. Alzheimer’s Dis. JAD 2017, 55, 321–331. [Google Scholar] [CrossRef]
- Camargos, E.F.; Louzada, F.M.; Nobrega, O.T. Wrist actigraphy for measuring sleep in intervention studies with Alzheimer’s disease patients: Application, usefulness, and challenges. Sleep Med. Rev. 2013, 17, 475–488. [Google Scholar] [CrossRef]
- Higami, Y.; Yamakawa, M.; Shigenobu, K.; Kamide, K.; Makimoto, K. High frequency of getting out of bed in patients with Alzheimer’s disease monitored by non-wearable actigraphy. Geriatr. Gerontol. Int. 2019, 19, 130–134. [Google Scholar] [CrossRef]
- Gietzelt, M.; Feldwieser, F.; Gövercin, M.; Steinhagen-Thiessen, E.; Marschollek, M. A prospective field study for sensor-based identification of fall risk in older people with dementia. Inform. Health Soc. Care 2014, 39, 249–261. [Google Scholar] [CrossRef]
- Di Rosa, M.; Hausdorff, J.M.; Stara, V.; Rossi, L.; Glynn, L.; Casey, M.; Burkard, S.; Cherubini, A. Concurrent validation of an index to estimate fall risk in community dwelling seniors through a wireless sensor insole system: A pilot study. Gait Posture 2017, 55, 6–11. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chung, P.C.J.; Wang, W.H.; Pai, M.C.; Wang, C.Y.; Lin, C.W.; Wu, H.L.; Wang, J.S. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014, 18, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Gietzelt, M.; Wolf, K.H.; Kohlmann, M.; Marschollek, M.; Haux, R. Measurement of Accelerometry-based Gait Parameters in People with and without Dementia in the Field. Methods Inf. Med. 2013, 52, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Hillel, I.; Shustak, S.; Del Din, S.; Bekkers, E.M.J.; . Pelosin, E.; Nieuwhof, F.; Rochester, L.; Mirelman, A. Everyday Stepping Quantity and Quality Among Older Adult Fallers With and Without Mild Cognitive Impairment: Initial Evidence for New Motor Markers of Cognitive Deficits? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Gillain, S.; Drame, M.; Lekeu, F.; Wojtasik, V.; Ricour, C.; Croisier, J.L.; Salmon, E.; Petermans, J. Gait speed or gait variability, which one to use as a marker of risk to develop Alzheimer disease? A pilot study. Aging Clin. Exp. Res. 2016, 28, 249–255. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Mulin, E.; Friedman, L.; Le Duff, F.; Cygankiewicz, E.; Deschaux, O.; Garcia, R.; Yesavage, J.A.; Robert, P.H.; Zeitzer, J.M. Decreased daytime motor activity associated with apathy in Alzheimer disease: An actigraphic study. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2012, 20, 806–814. [Google Scholar] [CrossRef]
- Kuhlmei, A.; Walther, B.; Becker, T.; Muller, U.; Nikolaus, T. Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2013, 28, 94–97. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; David, R.; Friedman, L.; Mulin, E.; Garcia, R.; Wang, J.; . Yesavage, J.A.; Robert, P.H.; Shannon, W. Phenotyping apathy in individuals with Alzheimer disease using functional principal component analysis. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2013, 21, 391–397. [Google Scholar] [CrossRef]
- Watts, A.; Walters, R.W.; Hoffman, L.; Templin, J. Intra-Individual Variability of Physical Activity in Older Adults With and Without Mild Alzheimer’s Disease. PLoS ONE 2016, 11, e0153898. [Google Scholar] [CrossRef]
- Kirste, T.; Hoffmeyer, A.; Koldrack, P.; Bauer, A.; Schubert, S.; Schröder, S.; Teipel, S. Detecting the effect of Alzheimer’s disease on everyday motion behavior. J. Alzheimer’s Dis. 2014, 38, 121–132. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nieto-Reyes, A.; Duque, R.; Montaña, J.L.; Lage, C. Classification of Alzheimer’s Patients through Ubiquitous Computing. Sensors 2017, 17, 1679. [Google Scholar] [CrossRef] [PubMed]
- Duque, R.; Nieto-Reyes, A.; Martínez, C.; Montaña, J.L. Detecting Human Movement Patterns Through Data Provided by Accelerometers—A Case Study Regarding Alzheimer’s Disease. In Proceedings of the Ubiquitous Computing and Ambient Intelligence—10th International Conference (UCAmI 2016), San Bartolomé de Tirajana, Gran Canaria, Spain, 29 November–2 December 2016; pp. 56–66. [Google Scholar] [CrossRef]
- Ann Ronao, C.; Cho, S.B. Human activity recognition with smartphone sensors using deep learning neural networks. Expert Syst. Appl. 2016, 59. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, W.; Oates, T. Time series classification from scratch with deep neural networks: A strong baseline. In Proceedings of the 2017 International Joint Conference on Neural Networks (IJCNN), Anchorage, AK, USA, 14–19 May. 2017; pp. 1578–1585. [Google Scholar] [CrossRef]
- Ioffe, S.; Szegedy, C. Batch Normalization: Accelerating Deep Network Training by Reducing Internal Covariate Shift. In Proceedings of the 32nd International Conference on International Conference on Machine Learning (ICML’15), Lille, France, 7–9 July 2015; Volume 37, pp. 448–456. [Google Scholar]
- Hinton, G.E.; Srivastava, N.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Improving neural networks by preventing co-adaptation of feature detectors. arXiv preprint 2012, arXiv:1207.0580. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. In Proceedings of the 3rd International Conference on Learning Representations (ICLR 2015), Conference Track Proceedings. San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
| Technique | Early-Stage. . | Middle-Stage. . | Late-Stage. . | Total |
|---|---|---|---|---|
| CNN | . . . . 89%. . | . . 93% | . . 91% | . . . 91% |
| MLP | . . . . 100%. . | . . 100% | . . 50% | . . . 83% |
| RT | . . . . 0%. . | . . 100% | . . 0% | . . . 50% |
| RF | . . . . 0%. . | . . 100% | . . 0% | . . . 50% |
| SVM | . . . . 0%. . | . . 100% | . . 0% | . . . 50% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bringas, S.; Salomón, S.; Duque, R.; Montaña, J.L.; Lage, C. A Convolutional Neural Network-Based Method for Human Movement Patterns Classification in Alzheimer’s Disease. Proceedings 2019, 31, 72. https://doi.org/10.3390/proceedings2019031072
Bringas S, Salomón S, Duque R, Montaña JL, Lage C. A Convolutional Neural Network-Based Method for Human Movement Patterns Classification in Alzheimer’s Disease. Proceedings. 2019; 31(1):72. https://doi.org/10.3390/proceedings2019031072
Chicago/Turabian StyleBringas, Santos, Sergio Salomón, Rafael Duque, José Luis Montaña, and Carmen Lage. 2019. "A Convolutional Neural Network-Based Method for Human Movement Patterns Classification in Alzheimer’s Disease" Proceedings 31, no. 1: 72. https://doi.org/10.3390/proceedings2019031072
APA StyleBringas, S., Salomón, S., Duque, R., Montaña, J. L., & Lage, C. (2019). A Convolutional Neural Network-Based Method for Human Movement Patterns Classification in Alzheimer’s Disease. Proceedings, 31(1), 72. https://doi.org/10.3390/proceedings2019031072






