Abstract
Photoplethysmography (PPG) is a non-invasive technique that employs near infrared light to estimate periodic oscillations in blood volume within arteries caused by the pulse pressure wave. Importantly, combined Electrocardiography (ECG) and PPG can be employed to quantify arterial stiffness. The capabilities of a home-made multi-channel PPG-ECG device (7 PPG probes, 4 ECG derivations) to evaluate arterial ageing were assessed. The high numerosity of channels allowed to estimate arterial stiffness at multiple body locations, without supra-systolic cuff occlusion, providing a fast and accurate examination of cardiovascular status and potentially allowing large scale clinical screening of cardiovascular risk.
1. Introduction
Cardiovascular insult is the main cause of death in developed countries [1]. However, cardiovascular risk assessment is challenging, because acute vascular events, such as stroke and infarction, often occur without a clear underlying cardiovascular pathology [1]. In clinical practice, cardiovascular status can be assessed through markers indicative of asymptomatic atherosclerosis based on the characteristics of the pulse pressure wave propagating through the arterial tree. Photoplethysmography (PPG) [2] is a noninvasive optical technique that exploits light in the near infrared (NIR) spectral range to measure blood volume oscillations induced by the pulse wave propagation from the heart within the peripheral arteries. With PPG, light is injected in the tissue and, after propagation within the biological structures, it is collected by a detector. The measured light intensity is sensitive to changes in volume of the arteries caused by the pulse pressure wave. PPG is often used in combination with electrocardiography (ECG) in order to synchronize the PPG waveform to the R-peak of the ECG trace. Several indices suggestive of cardiovascular risk can be computed from single pulse PPG signals. For example, the pulse wave velocity (PWV), the velocity at which the pulse pressure propagates within the arterial system, is known to be monotonically and positively associated with arterial stiffness [3]. Typically, its estimation requires two measurement locations (e.g., brachial-ankle PWV). Another index indicative of arterial stiffness and cardiovascular ageing is the augmentation index (AI). AI is calculated as the ratio between the augmentation pressure and the central pulse pressure (expressed in percentage) and it can be estimated through PPG [4]. Cardiovascular risk evaluation through PPG has several advantages when compared to pressure-based technologies. PPG measurements are simple and fast, operator independent and do not require supra-systolic cuff occlusion. However, commercial PPG technology is often equipped with few probes, not allowing a simultaneous investigation of the left and right hemi-body, thus increasing the examination duration and limiting the usage of PPG for broad clinical screening. In this study, a synchronized multi-channel PPG-ECG system is presented, and its capabilities of assessing cardiovascular ageing is investigated. The advantages of such a system rely on the simplicity and brevity of the measurements and on the possibility to simultaneously investigate different body sites. Specifically, PWV and AI, metrics associated to arterial stiffness, were computed from PPG signals collected on different body locations. Because of the known association between arterial stiffness and ageing, the relationships between age and the computed indices (PWV and AI) were inspected to validate the technology.
2. Material and Methods
2.1. Participants
41 healthy volunteers (Male/Female: 25/16; age: 20–70 years old) were enrolled in the study. All subjects were Caucasian, and they reported with no history of cardiovascular or psychiatric disease; they did not consume medications or drinks before the experiment, that was performed in agreement with the Helsinki Declaration and approved by the Ethical Committee of the local university. All the participants signed informed consent and could withdraw from the test at any time.
2.2. Multi-Channel PPG-ECG System
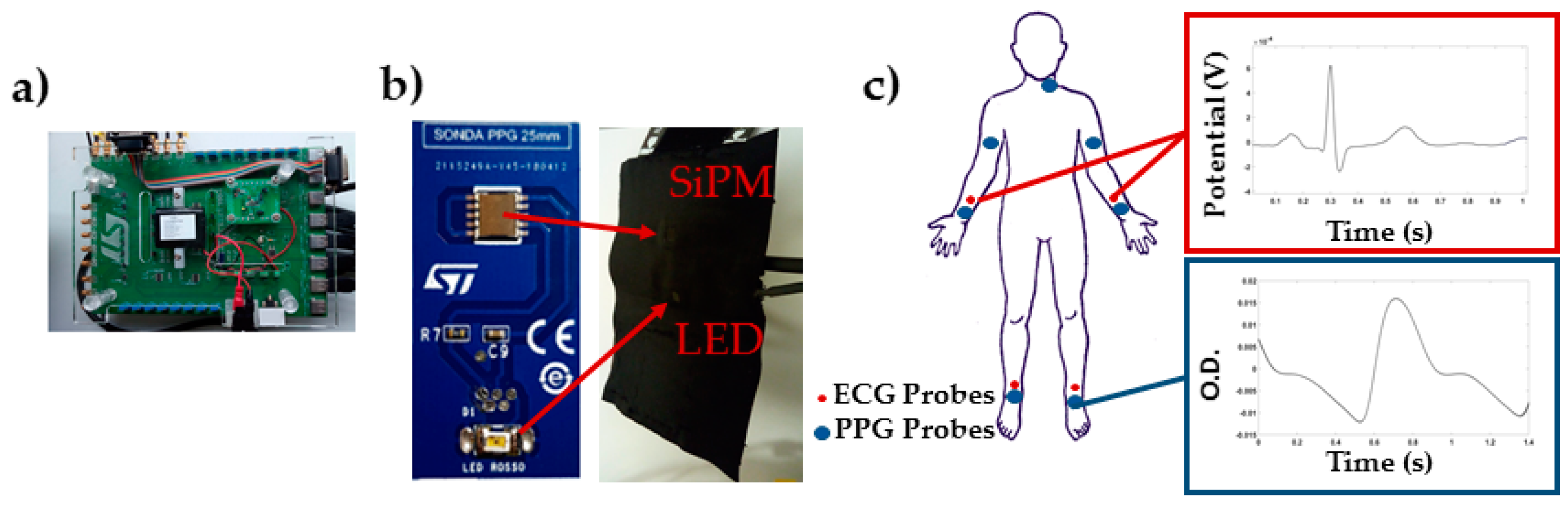
The PPG-ECG system was developed at STMicroelectronics (Figure 1a) and it was composed by 7 PPG probes (Figure 1b) and 4 ECG leads.
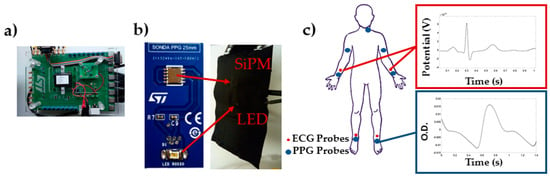
Figure 1.
(a) Printed circuit board for data acquisition; (b) Example of PPG probe; (c) Placement of the PPG probes and ECG electrodes, and example of average single location, single pulse ECG and PPG.
The PPG probes were composed by a Silicon-photomultiplier (SiPM) manufactured at STMicroelectronics [5,6] (detector), and a SMC940 LED (Roithner LaserTechnik, Vienna, Austria) emitting at 940 nm (light source). ECG signals were acquired by means of disposable ECG electrodes F2080 by FIAB. The PPG probes were interfaced with a NI PXIe4303 (National Instruments, Austin, TX, USA) acquisition instrumentation by means of a specific printed circuit board (PCB). The PPG and ECG signals were acquired simultaneously with a sampling frequency of 1 kHz. A LabVIEW software program was developed to control the acquisition system for data collection.
2.3. Experimental Design
PPG probes were placed over the carotid (left), brachial, radial/ulnar, and tibial (left and right) arteries (Figure 1c). The sensors were held in place using custom-built probes equipped with pressurized cuffs (80 mmHg) to support the sensors-skin optical coupling. During the experiment, the subjects lied supine on a medical cot. PPG and ECG data were recorded for 30 s.
2.4. PPG-ECG Data Analysis
Raw PPG signal was converted into optical densities (OD). Raw OD and ECG were filtered employing a band-pass 4th order Butterworth digital filters (PPG signal, cut-off frequencies: 0.2 and 10 Hz; ECG signal, cut-off frequencies: 0.2 and 50 Hz). The R-wave peaks identification was performed by searching for the local maxima on filtered and normalized (z-score) Lead I ECG signals. Single pulse PPG was estimated for all channels in a time window from 0.3 s prior to 1.5 s after the R-wave peaks. A trim-mean approach for the average pulse evaluation was applied to remove noisy signal periods. On the average PPG pulse, brachial-tibial and ulnar-tibial PWVs and AI for all the measurement sites were computed as follow:
where Δx is the distance between two measurement locations and Δt is the time delay between the diastolic foots of the PPG signals between the two locations [3]; AP is the augmentation pressure (difference between the systolic peak and the following peak) and PP is the central pressure (difference between the systolic peak and diastolic foot) [4]. To test the ability of multisite PPG to assess cardiovascular ageing, the correlations between AI and age and PWV and age were evaluated.
3. Results
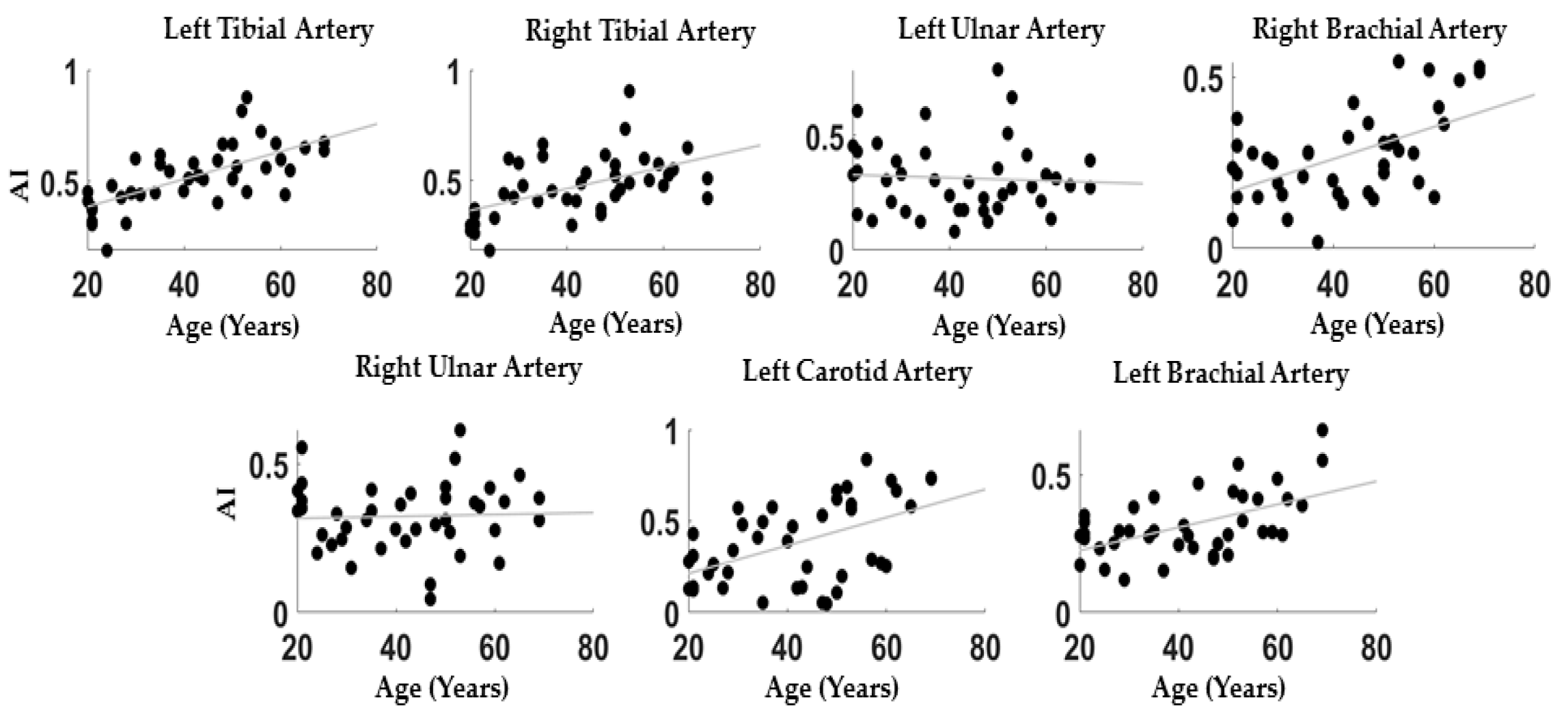
Figure 2 reports the correlations between AI and age evaluated at the different PPG body sites: Left Tibial: r = 0.67; p = 4 × 10-5; Right Tibial: r = 0.52; p = 5 × 10-4, Left Ulnar: r = −0.06; p = 0.71, Right Ulnar: r = 0.04; p = 0.80, Carotid: r = 0.50; p = 8 × 10-4, Left Brachial: r = 0.54; p = 3 × 10-4, Right Brachial: r = 0.54; p = 3 × 10-4.
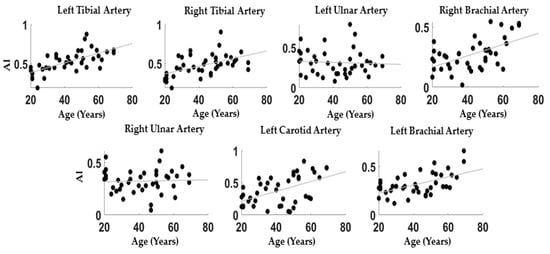
Figure 2.
Scatter plots between age and AI for all the measurement locations.
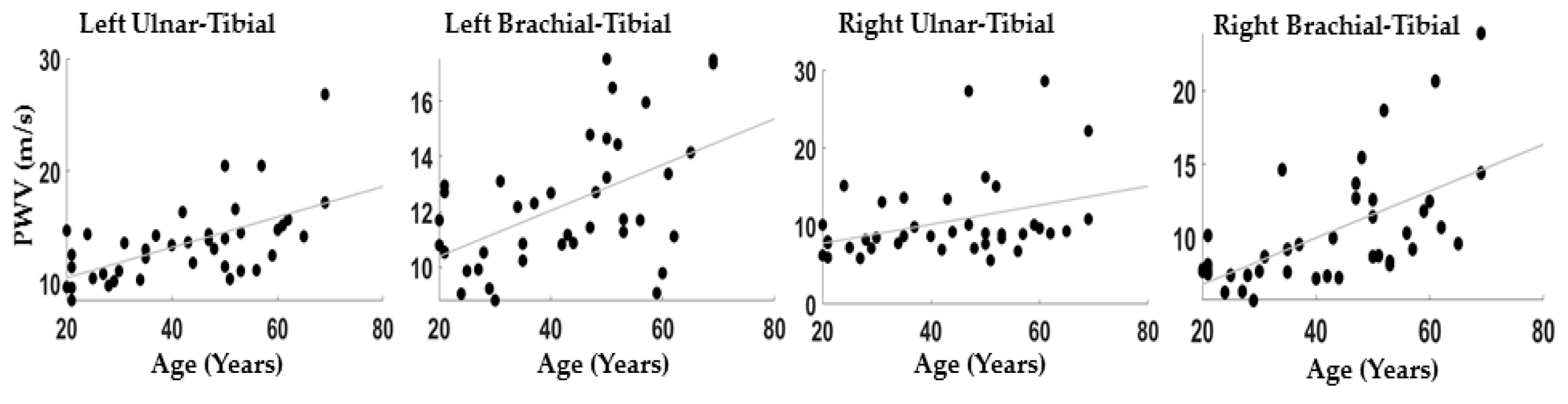
Figure 3 reports the correlations between the PWV evaluated between brachial-tibial and ulnar-tibial arteries on the right and left side of the body: Left Brachial-Tibial: r = 0.52; p = 5 × 10−4; Right Brachial-Tibial: r = 0.59; p = 1 × 10−4; Left Ulnar-Tibial: r = 0.60; p = ~0; Right Ulnar-Tibial: r = 0.35; p = 0.03.
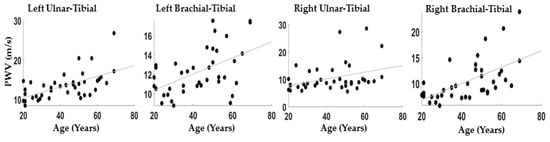
Figure 3.
Scatter plot between age ad PWV for all the couples of channels considered.
4. Discussion
Because of the high incidence of cardiovascular insults, prevention and control are of great importance. For this reason, several markers of cardiovascular risk were developed, such as AI and PWV. The feasibility of assessing AI and PWV using a home-made multi-channel PPG-ECG system was here demonstrated. The multi-channel PPG system allowed simultaneous recording from different body locations, providing an accurate and localized investigation of arterial status. Moreover, it permitted to perform cardiovascular assessment in a very short time (~30 s), important feature for clinical applications. The results suggested that a multi-channel approach is suitable for assessing vascular ageing. In fact, the correlations between age and AI and PWV are generally above 0.5. The found correlations between age and parameters of vascular ageing are comparable to the values reported in literature [2,7]. However, for some body locations the correlation between these parameters and ageing are not significant. These findings confirm the importance of employing a multi-channel configuration. The obtained results suggest that tibial and brachial arteries provide more information concerning vascular ageing with respect to ulnar and carotid arteries through PPG. However, it is not possible to exclude that, for some cardiovascular pathologies, PPG on ulnar and carotid arteries may provide important information regarding vascular status. One limitation of the study is indeed the reduced sample size and its homogeneity (only healthy people were included). Moreover, channels’ information was analyzed separately, without accounting for possible synergistic effects between body locations that could be highlighted by multivariate analysis. Further studies are indeed necessary to better investigate the clinical valence of a multi-channel PPG instrumentation for cardiovascular risk assessment by increasing the sample size, including pathological subjects and by implementing multivariate approaches. In conclusion, the results demonstrated the significance of multisite PPG for cardiovascular assessments that could become a powerful screening tool in clinical practice.
Author Contributions
Conceptualization, D.P., A.M.C., F.B., S.G., P.V., S.R., V.P., G.F., A.M.; methodology, D.P., P.V., F.B., S.R., S.G., V.P.; hardware, G.F., V.V.; formal analysis, D.P., D.C., G.F.; writing—original draft preparation, D.P.; writing—review and editing, D.P., A.M.C.; supervision, A.M.; project administration, A.M.; funding acquisition, V.V., A.M.
Funding
This research was partially funded by grant H2020, ECSEL-04-2015-Smart Health, grant n. 692470, Advancing Smart Optical Imaging and Sensing for Health (ASTONISH).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review - ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0021915006001390 (accessed on May 16, 2019).
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Nandini, H.; Pandey, A.K. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Int. Arch. BioMed Clin. Res. 2018, 4, 29–31. [Google Scholar]
- Pilt, K.; Meigas, K.; Ferenets, R.; Temitski, K.; Viigimaa, M. Photoplethysmographic signal waveform index for detection of increased arterial stiffness. Physiol. Meas. 2014, 35, 2027. [Google Scholar] [CrossRef] [PubMed]
- Mazzillo, M.; Ronzhin, A.; Los, S.; Abbisso, S.; Sanfilippo, D.; Valvo, G.; Carbone, B.; Piana, A.; Fallica, G.; Albrow, M. Electro-optical performances of p-on-n and n-on-p silicon photomultipliers. IEEE Trans. Electron Devices 2012, 59, 3419–3425. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Ambra, E.; Maddiona, L.; Oliveri, S.; Romeo, M.F.; Mazzillo, M.; Rundo, F.; Fallica, G. Progresses towards a processing pipeline in photoplethysmogram (PPG) based on SiPMs. In Proceedings of the 2017 European Conference on Circuit Theory and Design (ECCTD), IEEE, Catania, Italy, 4–6 September 2017; pp. 1–5. [Google Scholar]
- Millasseau, S.C.; Kelly, R.P.; Ritter, J.M.; Chowienczyk, P.J. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin. Sci. 2002, 103, 371–377. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).