Abstract
We report an easy-to-use Lab-on-Chip (LoC) device able to detect soluble, circulating biomarkers in plasma that are relevant to Coronary Artery Disease (CAD). The LoC prototype is developed within the SMARTool European project and is intended to be used for Point-of-Care (PoC) testing of patients with CAD, facilitating more rapid and efficient monitoring and treatment decisions. A LoC prototype is presented, enabling chemiluminescent assays to be performed on chip targeting biomarkers relevant to CAD. In parallel, a robust technology for electrostatically actuated, capillary burst valve for PoC applications, integrated in potentially disposable, thermoplastic devices is reported. The devices were fabricated using easily scalable fabrication techniques and can be used to perform multistep assays on single-use microfluidic devices.
1. Introduction
Lab on chip technologies for point-of-care (PoC) testing have attracted wide attention over the last years. Their growth has been fueled by the increasing need for developing simple to use and cost-effective microfluidic tools for decentralized diagnostic testing. More particularly, due to the need of using low reagent volumes and performing multiple fluidic analysis in parallel, there is a growing interest in miniaturizing and integrating complete biological assays on single chips. PoC devices are usually employed to detect biomarkers that are indicators of specific diseases [1]. A considerable amount of PoC devices that have been reported, are based on immunoassays and aim at detecting the antigen/biomarker of interest [2].
The work presented in this paper is part of the SMARTool European project. The objective of this work is to yield a prototype targeting PoC testing and try to reduce the gap distance between research and clinical practice, performing on chip operations that usually require dedicated machinery (e.g., flow cytometers). More specifically, a microfluidic prototype for selective capturing of CAD-related biomarkers is presented, as a cost-effective improvement of current clinical strategies for diagnosis and prognosis of CAD. The developed module of the lab on chip device focuses on the detection of soluble biomarkers and more specifically proteins present in plasma and which can be relevant for CAD investigations. For the detection and quantification of these biomarkers, a dedicated device was fabricated. An optical, chemiluminescent-based, detection system was selected to be integrated on the device. In this way, no excitation source and additional filters are needed and the device can be potentially easily miniaturised and low cost. A protocol for performing an Enzyme-Linked Immunosorbent Assay (ELISA) on chip was established. Further investigation is carried out in order to propose going towards a device with minimal external ancillary equipment, based on capillary flow control that can quantify the presence of CAD related biomarkers.
2. Materials and Methods
Cyclin Olefin Copolymer (COC) was the selected substrate to fabricate the disposable devices. COC is a polymer that has low water absorption, is resistant to solvents such as iso-propyl alcohol and acetone and has good optical properties. These are important characteristics for point of care applications.
2.1. ELISA-on-Chip Detection Platform
The first set of devices was developed in order to be able to establish the ELISA-on chip protocol for the detection of CAD-related biomarkers, such as the ICAM-1 protein. ICAM-1 protein is implicated in the role of recruiting inflammatory cells and in combination with lipids and lipoproteins is responsible for atherosclerosis. The first set of chips was fabricated by micro-milling. The dimensions of the microfluidic chip that is designed for performing the ELISA are 15 × 45 mm. Each chip allows the performance of two assays and consists in two inlets, two outlets and two detection chambers (Figure 1). The volume of the liquid that can be contained in the detection chamber is 0.75 μL.
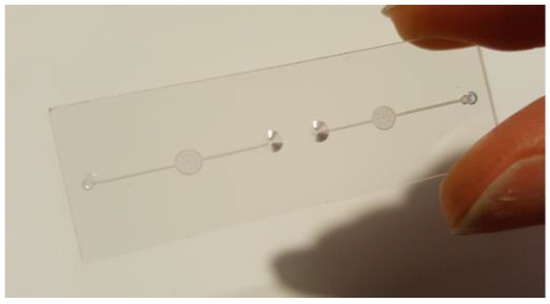
Figure 1.
ELISA on a chip device.
The type of ELISA employed for this module is the sandwich ELISA. The antigen, which is the ICAM-1 protein, is captured between two layers of antibodies, the capture and detection antibodies. A standard ELISA kit for the detection of Recombinant Human ICAM-1 was purchased from R&D systems and adapted to a microfluidic format. The anti-human ICAM-1 capture antibodies were first immobilized on the surface of the open COC chip, relying on protein adsorption on the hydrophobic plastic surface of the device. Once the functionalization process is completed, the chip was sealed using a layer of COC foil (100 μm). The COC foil was stuck on the underlying chip with a 3MTM optically clear adhesive (25 μm).
The chip was then placed in the clamping system, the Fluidic Connect Pro system developed by Micronit. An optical Complementary Metal–Oxide–Semiconductor (CMOS) sensor, provided by Anitoa Systems, was integrated on the system and could measure the chemiluminescent signal emitted when the ELISA was completed. The whole system was connected to the syringe pump through the outlet tubing (Figure 2a). Figure 2b presents a schematic cross section of the microfluidic chip. The inlets are tapered so that the liquid can be funneled in a better way into the chip. The operating principle simply lies in filling in the inlet with the liquid using a pipette, letting the liquid go through the detection chamber and withdrawing it from the outlet using the syringe pump. Walls of the channel and chamber were then functionalized by blocking all the non-specific binding sites on the channels and chamber, using a solution of 1% BSA in PBS (surface passivation step), with a flow rate of 0.5 μL/min.
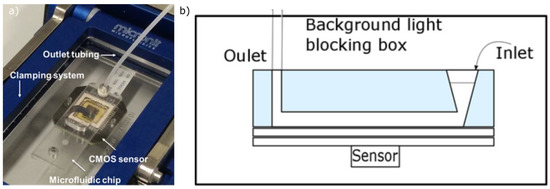
Figure 2.
(a) ELISA set-up including the microfluidic chip, the CMOS sensor and the outlet tubing, all of them brought together through the clamping system; (b) Schematic cross section of the microfluidic chip during the detection step.
After these preparations, the first step of the assay was to insert the antigen (Recombinant Human ICAM-1 Standard in the detection chamber. The antigen binds to the capture antibodies that are pre-coated in the chamber. A washing buffer, consisting in a solution of 0.05% Tween® 20 in PBS, was then used to remove the unbound antigen. A solution containing the biotinylated anti-human detection antibodies (Sheep Anti-Human ICAM-1) was inserted in the detection chamber and the biotinylated detection antibodies bind to the already captured antigen. The unbound antibodies were removed by flushing in washing buffer. The next step was to insert a solution containing streptavidin, conjugated to HRP. The affinity of streptavidin for biotin is a very strong non-covalent biological interaction and therefore the streptavidin binds to the biotin already present on the antibodies. The unbound streptavidin was removed by flushing washing buffer. The final step consisted in introducing the chemiluminescent substrate, Lumi-Phos Atto, in the detection chamber. The total assay time is around 1 h which is considerably shorter than the assay time suggested for the well-plate format. Moreover, the volumes of the costly reagents needed to perform the assay are dramatically reduced since the experiment is transferred to the microscale.
2.2. Microfluidic Devices for Integrated Flow Control
In parallel to the ELISA-on-chip system development, a second set of devices was fabricated for investigating a capillary-based flow control system. This system can be later integrated in the ELISA-on chip platform to enable performing the assay with minimal external pumping equipment.
The structures were embossed on COC substrates. For the soft embossing process, a dry film resist was used to pattern structures on silicon master mold. The intermediate PDMS mold was then fabricated for embossing COC substrates. Gold microelectrodes were deposited and patterned on different COC substrates, through sputtering and photolithography processes. The two substrates, containing the embossed structures and electrodes, were bonded together using a Collin platen press.
3. Results and Discussion
3.1. Biomarkers Detection through the ELISA-on-Chip Detection Platform
The CMOS sensor records the chemiluminescent signal emitted during the final step of the assay. The readout of the sensor is automatically exported in a text file, which was analyzed using a custom Matlab script. The script imports the data file and extracts the intensity levels for each frame. Figure 3 shows the results of the Matlab analysis before the quantification. The frame at 2 s shows the signal detected by the sensor before the Lumi-Phos arrives at the detection chamber while the image at 10 s corresponds to the frame taken when the Lumi-Phos is in the detection chamber and interacts with the anti ICAM-1 antibodies that are immobilized on the surface.

Figure 3.
The result of Matlab analysis before the quantification. Frame at 2 s corresponds to the moment before the Lumi-Phos has arrived at the detection chamber while the frame at 10 s shows the signal detected when the Lumi-Phos is in the chamber and interacts with the anti ICAM-1 antibodies that are immobilised on the surface.
The concentration range tested for ICAM-1 Standard was from 0 to 50 ng/mL which covers the concentration range that can be found for a healthy subject. The quantification result of the experiment is a curve reporting the measured luminescence as a function of time and is presented in Figure 4. The curve can be divided in three parts: (1) Initial signal increase, corresponding to the arrival of the Lumi-Phos in the detection chamber. The signal increases until an equilibrium is reached between the consumption of luminescent solution from the HRP, and the new substrate introduced in the chamber by the fluid flow; (2) When the equilibrium is reached, the signal reaches a plateau. Slow deflections from this constant value can be attributed to the HRP non-specifically adsorbed to the walls of the chip, that slowly reduce the concentration of chemiluminescent substrate in the channel. The maximum value reached during the plateaux is directly proportional to the amount of HRP (and therefore of analyte) in the chamber; (3) The flow is then stopped. Without providing fresh substrate, the enzyme depletes all the Lumi-Phos, and the signal rapidly decreases over time.
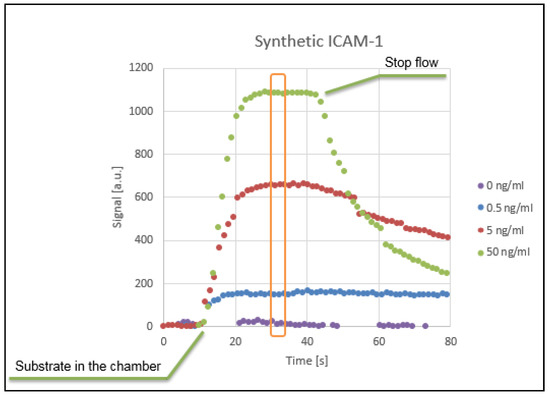
Figure 4.
Signal intensity recorded by the integrated optical sensor, plotted versus time for different ICAM-1 concentrations.
Based on the maximum values reached during the plateau, the calibration curve was established and is shown in Figure 5.
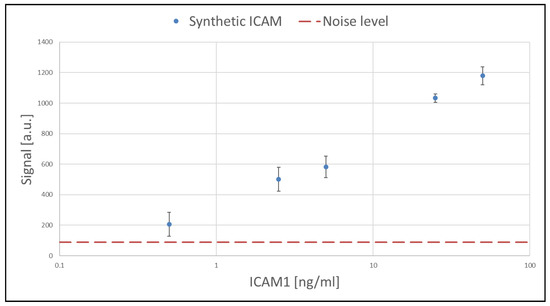
Figure 5.
Calibration curve for the detection of synthetic ICAM-1 standard protein. The concentration range covered is 0–50 ng/mL.
Overall, the on-chip detection shows good linearity (in logarithmic scale) over two orders of magnitude of concentration of the synthetic target (calibration curve), overlapping with the concentration range of ICAM-1 in real blood plasma samples of healthy subjects and patients.
3.2. Integrated Flow Control System
In order to be able to perform an ELISA on chip, a fundamental element that needs to be assured is the mechanism to obtain a controlled fluidic sequence. The flow control can be either assured by external means (using for instance syringe pumps) or with integrated functional elements.
Targeting an autonomous and portable device, it is important to minimize the need for external pumping equipment and integrate elements that can control the movement of the fluid inside the channels. To fulfil this requirement, the fluidic movement is based on capillary action. It is tuned by developing the appropriate surface functionalization strategy and is controlled combing capillary burst valves and the electrostatic actuation mechanism.
The capillary burst valves can stop the fluid flow. They are based on a sudden expansion of the microfluidic channel that stops the capillary filling (Figure 6).

Figure 6.
Capillary stop valves embossed on COC substrates. The valves are based on a sudden expansion of the cross section of the channel.
Electrostatic actuation of capillary burst valves is an effective way of controlling fluidic movement inside the channels and is required to develop a reliable, multistep, on-chip assay. The operation principle lies into applying a voltage between two electrodes that are placed before and after the valve in order to attract the meniscus towards the second electrode and trigger the valve. Both functional elements, capillary valves and metal electrodes were integrated in a microfluidic chip since they use little power and are therefore suitable for use in handheld instruments (Figure 7). These initial devices were characterized and the triggering voltage for actuating capillary valves that hold a pressure of 30 mbar, was determined to be in the kV range, tuneable by electrode geometry and valve strength. Since actuation is by electrostatic means—not to be confused with electrowetting, little power is consumed and the use of short duration pulses prevent electrochemical processes that occur at the electrodes once the liquid makes contact with the counter electrode.
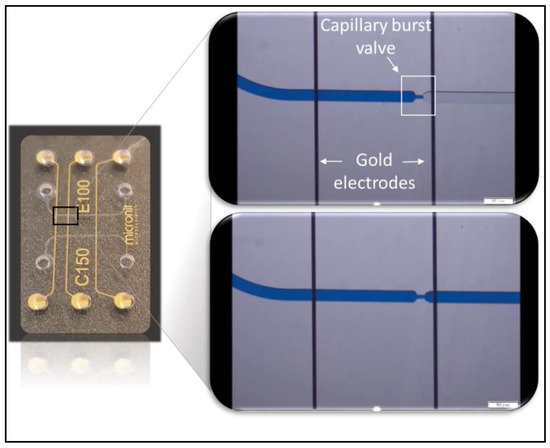
Figure 7.
COC device integrating capillary burst valves and electrodes aimed at the triggering of the valves. The top right image shows the meniscus stopped at the valve and is taken before applying the voltage between the two electrodes. The bottom right image is taken after voltage application and therefore triggering of the valve.
The integration in microfluidic devices of the developed functional elements, gives the possibility of performing multistep assays on single-use microstructured devices with improved temporal and spatial control, mainly targeting point-of-care applications. This system, after further fine tuning, can be integrated on the LoC devices enabling ELISA-based determination of the biomarkers concentration.
4. Conclusions
An easy-to-use microfluidic prototype for detecting circulating biomarkers related to Coronary Artery Disease (CAD) diagnosis and/or prognosis is reported in this article. It was demonstrated that the ELISA protocol can be transferred from the micro-well plate format to the microfluidic format. The prototype, consisting in the microfluidic chip placed in the clamping system and the integrated optical sensor, was tested on synthetic samples for the detection of the ICAM-1 protein, a biomarker related to the presence of CAD. Overall, the on-chip detection showed good linearity (in logarithmic scale) over two orders of magnitude of concentration of the synthetic target, overlapping with the concentration range of ICAM-1 in real blood plasma samples of healthy subjects and patients. In parallel, a strategy was investigated to develop an integrated flow control system based on capillary flow, aiming at eventually minimizing the requirements in terms of ancillary apparatus and human-operation.
Author Contributions
A.T. and S.M. conceived, designed, performed and analyzed the experiments; S.R., G.P. and M.B. contributed to the conception of the experiments and the interpretation of the results.
Acknowledgments
This project has received funding from the EU H2020 research and innovation program under grant agreement No. 689068. The authors would also like to thank Anitoa Systems for providing the CMOS sensor.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results”.
References
- Liu, C.; Qiu, X.; Ongagna, S.; Chen, D.; Chen, Z.; Abrams, W.R.; Malamud, D.; Corstjensd, P.L.A.M.; Bau, H.H. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip 2009, 9, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Zirath, H.; Schnetz, G.; Glatz, A.; Spittler, A.; Redl, H.; Peham, J.R. Bedside Immune Monitoring: An Automated Immunoassay Platform for Quantification of Blood Biomarkers in Patient Serum within 20 Minutes. Anal. Chem. 2017, 89, 4817–4823. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).