Abstract
Molecular tunneling process has been considered by means of radiation theory. The formula for information entropy calculation has been derived by means of interaction model of thermal equilibrium radiation with a molecule at low temperatures. The physical meaning of information entropy for low-temperature plateau of unimolecular chemical reaction has been determined. It is a measure of conversion of thermal radiation energy to mechanical energy that moves atoms in a molecule during elementary activation act. It is also a measure of uncertainty of this energy conversion. The conversion takes place at a temperature when the average energy of the elementary activation act is equal to a part of zero energy of the transforming molecule. Two unimolecular reactions have been investigated. These are Fe-CO bond recombination in β-hemoglobin and double proton transfer in benzoic acid dimer for sequential deuteration of hydrogen bond and various hydrostatic pressures. Using the information entropy formula it is possible to calculate its value in energy units of measurements for low-temperature plateau. Probabilities of occurrence of the reactions under considerations, their efficiency and mean-square fluctuations of the distribution function parameters have also been determined.
1. Introduction
The activation process model was proposed in 1990 [1], its physical nature being alternative to the well-known Boltzmann-Arrhenius model. As a result of its development, the interaction model of thermal equilibrium radiation with a molecule at low temperatures was created in 2002 to explain such a puzzling process as molecular tunneling [2]. The interaction process was caused by subsequent realization of the molecule’s sub-conformations. Each sub-conformation state was approximated by means of a harmonic quantum oscillator. The oscillator was considered non-ideal, i.e., its degeneracy and exclusion of some excited vibration levels was taken into account. The molecular tunneling itself was due to a part of the oscillator’s zero energy, the energy being the result of the transition from the previous sub-conformation state to the next one. All the process features were allowed for while choosing the distribution function parameters. This made possible a numerical simulation of four molecular tunneling reactions. These were photoinduced conversion of rhodopsin and deuterated rhodopsin into prelumirhodopsin, low-temperature polymerization of formaldehyde, and Fe-CO bond recombination in β-hemoglobin. Information entropy of activation process has been derived recently [3]. Its physical meaning has also been determined. It would be useful to do the same for molecular tunneling process, since several researchers believe that information entropy is conceptually more fundamental even by comparison with energy [4]. So in this paper we derive the formula for information entropy of molecular tunneling. Then two reactions are investigated numerically. These are Fe-CO bond recombination in β-hemoglobin and double proton transfer in benzoic acid dimer. The second reaction is considered for sequential deuteration of hydrogen bond and for various hydrostatic pressures. For all these reactions the values of information entropy are calculated. Probabilities of occurrence of the processes under consideration, their efficiency and mean-square fluctuations of the distribution function parameters are also determined.
2. Information Entropy of Molecular Tunneling
According to the activation process model [5], the activation barrier for molecular tunneling process can be represented as a set of m identical quantum oscillators (Figure 1). These oscillators denote intermediate sub-conformations of a molecule as the latter transforms. The barrier is in thermodynamic equilibrium with the thermal radiation. Any pair of adjacent oscillators has their energy levels shifted by μhν. Here h is Planck constant, μ is the parameter describing the levels shift, and ν is the effective frequency of the quantum oscillator averaged over the generalized reaction coordinate. The value of ν equals to the Einstein spontaneous emission coefficient A [6]. The probability of a transition over this activation barrier can be written as [2]
where Ω is the degree of degeneracy for (n + μ) hν vibration level.
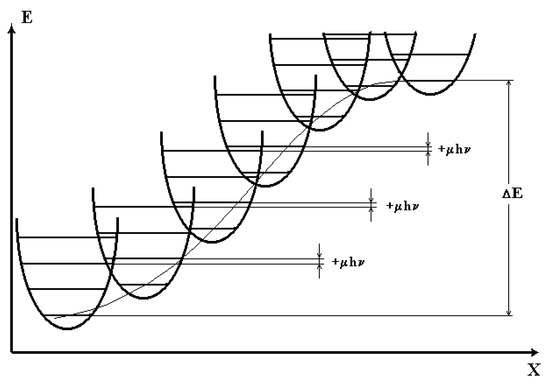
Figure 1.
Arrangement of the quantum oscillators averaged over the generalized coordinate of chemical reaction on the potential barrier.
Consequently, information entropy for the process under consideration can be expressed by means of energy units of measurement [3]
Now, let us take into account the fact that different levels (or groups of levels) in the quantum oscillators can have the different degrees of degeneracy, e.g., Ω1, Ω2, Ω3 (Figure 2), i.e., the vibration levels up to quantum number ξ − 1 are non-degenerate, the ones from quantum numbers ξ to η − 1 have the degeneracy Ω1, and those from η to ζ − 1, Ω2, and from ζ to ∞, Ω3 [5,7]. The transition is forbidden if Ω = 0.
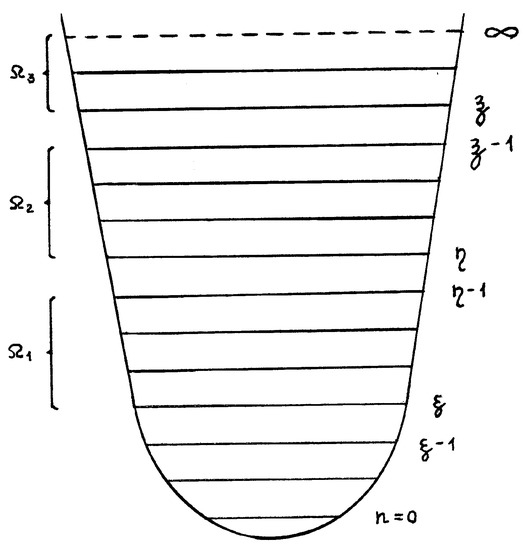
Figure 2.
Partition of the energy levels of the averaged quantum oscillator taking into account of the degree of degeneracy (ξ < η < ζ).
Considering the levels partition, the denominator of (1) may be presented as
On the other hand, this denominator can be simplified analytically [2,7]
where and .
So Equation (1) can be transferred into
Entropy is additive, so (2) can be separated into four terms:
where
These four terms can be calculated using the technique described in [3], so we only write the final expression for information entropy:
with and .
It should be noted there is no parameter μ responsible for molecular tunneling in (3). Let us try to overcome this apparent misunderstanding in the next section.
3. Information Entropy of Low-Temperature Plateau for Fe-CO Bond Recombination in β-Hemoglobin
Such a process can be simulated with a set of quantum oscillators with non-degenerate vibration levels [2], that is Ω1 = Ω2 = Ω3 = 1. It is the distinctive feature of this reaction that allows one to simplify (3) as
Equation (4) was derived in the previous paper [3]. By multiplying both parts of (4) into T/m, we get
The first term of (5) defines the average energy of elementary activation act. The second one describes a measure of energy uncertainty per quantum subsystem, i.e., a molecule’s sub-conformation. It is known from experiments there always exists a temperature of transition from a low-temperature plateau to an activation part for any Arrhenius dependence. Solely from the above reasoning we write the following equality:
Hence the temperature of transition T1 can be found as
Information entropy can be expressed in terms of μ only. To do this, we substitute (7) into (5):
The following values of the parameters for the reaction under consideration can be used: ν = 3.3·106 Hz, m = 9.0 × 105, μ = 1.8·106, h = 6626 × 10−34 J s, and k = 1381 × 10−23 J/K [2]. Insertion of these values into (7) gives T1 = 10.776 K. Now it is possible to calculate the value of information entropy for the beginning of activation part of experimental Arrhenius dependence. To do this, we substitute the value of T1 into (4) to get Sim = 3.536 × 10−28 J/K.
It should be noted that entropy Sim is measured in energy units. On the other hand, (8) gives the value of information entropy for the low-temperature plateau = 3.536 × 10−28 J/K. Comparison of the two values shows that Sim is the required parameter corresponding to the low-temperature plateau.
Notice that T1 is approximately equal to the temperature of activation barrier creation Tm = 10.397 K [8]. Besides, Laura Eisenstein found experimentally that a chemical reaction rate constant of Fe-CO bond recombination in β-hemoglobin is temperature independent for temperatures lower than 10 K [9]. All things considered, the interaction model of thermal equilibrium radiation with a molecule at low temperatures is realistic for molecular tunneling representation. So we have examined the ideal example of molecular tunneling reaction, i.e., when an activation barrier can be represented as a set of oscillators with non-degenerate vibration levels. Let us consider another reaction where the degeneracy and exclusion of some excited vibration levels will be taken into account.
4. Information Entropy of the Double Proton Transfer in the Benzoic Acid Dimer
This process was numerically simulated in [5]. The following formula for the average energy of the elementary activation act was used:
By analogy with (6) we can write a transcendental equation:
One has to solve (10) numerically to get the temperature of transition T1 from the low-temperature plateau to the activation part of Arrhenius dependence. Substitution of T1 into (3) gives the value of information entropy for the low-temperature plateau of the process under consideration.
Experimental studies of the temperature dependence of the chemical reaction rate constant were made for monocrystalline simples with sequential deuteration of hydrogen bonds [10] and polycrystalline ones under various hydrostatic pressures [11].
4.1. Sequential Deuteration of the Hydrogen Bond
4.1.1. Information Entropy of the Low-Temperature Plateau
Numerical simulation of this process showed the parameters Ω3 and ζ are excess [5]. So (10) can be rewritten as
The calculated values of T1 can be found in Table 1.

Table 1.
Information entropy for partial and complete deuteration of the hydrogen bond in the benzoic acid dimer.
Equation (3) may be simplified here because :
The derived values of information entropy are shown in Table 1.
Now let us return to the ideal set of oscillators with non-degenerate vibration levels. This set is a simplification for our deuteration process, but we can use (8) to get the values of and compare them with those of . As seen from Table 1, are 3–20 times smaller than . This result can be reasonably expected.
4.1.2. Assumptions Needed for Calculation of the Required Parameters
To calculate probabilities of occurrence of the reactions under consideration as well as their efficiency and mean-square fluctuations of the entropy parameters, one has to know two temperatures [3]. These are the temperature of activation barrier creation Tm and the temperature T2 when the barrier is overcome by absorbing only two quanta of thermal equilibrium radiation (TER). These temperatures were derived analytically for the set of ideal quantum oscillators [8]. The corresponding activation barrier was in quasi-equilibrium with TER. Temperature independent Einstein coefficients A and B were used to get the values of T2 and Tm. However, these coefficients depend on temperature in our case, so it is not possible to write down a set of equations that satisfy the Einstein detailed balancing principle for a molecule interacting with TER. One has to invoke some other assumptions to calculate Tm and T2. Such assumptions must not be contradictory to a physical meaning of the activation process model.
It has just been found in Section 3, that Tm is approximately equal to T1 (Tm = 10,391 K vs. T1 = 10,776 K), where T1 is the temperature of transition from the low-temperature plateau to the activation part for the Arrhenius dependence of Fe-CO bond-recombination in β-hemoglobin. Let us assume that T1 is the temperature of activation barrier creation if the barrier consists of non-ideal quantum oscillators. Of course, this assumption requires a more rigorous proof. Nevertheless, we can state that at T1 the activation barrier begins to manifest itself by defining the chemical reaction rate constant of the process under consideration.
To get the value of T2, the following reasonings must be taken into account. First, as the molecule under transformation is in thermodynamic quasi-equilibrium with TER, its probability of transformation should be slightly less or greater than 0.5 [3]. Second, mean-square fluctuations of the distribution function parameters must be less than parameter values themselves, for the distribution function has no physical meaning otherwise.
So the change of information entropy of activation that defines the experimental Arrhenius dependence can be derived using (12):
Suppose that a unimolecular reaction is a large thermodynamic equilibrium fluctuation. Then we can calculate its probability of occurrence using Einstein Formula [12,13]
To get a unimolecular reaction efficiency, both left-hand and right-hand sides of (12) should be multiplied by T/m
The first term of the right-hand side of (15) is the average energy of the elementary activation act [3,5]. The two other terms describe uncertainty of this energy. The uncertainty is due to unevenness and fuzziness of the distribution function representing the activation process. If the left-hand side of (15) is taken to be 100%, a unimolecular reaction efficiency can be easily calculated.
So if a unimolecular reaction can be represented as a large fluctuation, then information entropy change is a consequence of this fluctuation. Knowing one can get mean-square relative fluctuations of the entropy parameters. The following formula proposed by Einstein [12] can be used
where Δ is one of the parameters that determines the entropy change as a result of the fluctuation.
4.1.3. Calculation Results

Table 2.
Parameters for sequential deuteration of the hydrogen bond in the benzoic acid dimer.

Table 3.
Comparison of the average energy of the elementary activation act and its uncertainty for sequential deuteration of the hydrogen bond.
In Table 2 is the effective activation frequency. As seen from the results, there exists a correlation between and . The correlation can be written as When calculating T2 and W, the results turn out to be very sensitive to ξ. This parameter describes the lowest vibration level of harmonic oscillator where a quantum sub-conformation of the transforming molecule can be called non-ideal.
Table 3 demonstrates the efficiency of the reactions under consideration.
As temperature increases, so does the reaction efficiency. This result is somewhat unexpected, since the temperature dependence of Fe-CO bond recombination in β-hemoglobin was shown to be just the opposite [3].
When the results of Table 3 are compared with those of Table 4, it is apparent that a unimolecular reaction efficiency increases with increasing temperature if the reaction is represented as a set of non-ideal quantum oscillators.

Table 4.
Reaction efficiency of Fe-CO bond recombination in β-hemoglobin for various temperatures.
4.2. Information Entropy of the Double Proton Transfer in the Polycrystalline Benzoic Acid Sample under Various Hydrostatic Pressures
As in the previous section, the activation barrier can be represented as a set of ideal or non-ideal quantum oscillators. The corresponding values of information entropy for low-temperature plateaus are given in Table 5. It can be easily seen that are 2–3 times smaller than .

Table 5.
Information entropy of the double proton transfer in the polycrystalline benzoic acid sample under various hydrostatic pressures.
Square roots of mean-square relative fluctuations of the distribution function parameters are shown in Table 6. Once again, there is a correlation between the effective activation frequency and oscillator frequency . It can be written as

Table 6.
Parameters for the double proton transfer in the polycrystalline benzoic acid dimer under various hydrostatic pressures.
The value of is still extremely important when calculating T2 and W.
The reaction probability decreases with increasing hydrostatic pressure applied to the sample. The pressure dependence is attributable to the departure of the thermodynamic system under consideration from the equilibrium state, so that the probability is less than 0.5.
Once again, as temperature increases, so does the reaction efficiency (Table 7).

Table 7.
Comparison of the average energy of the elementary activation act and its uncertainty for various hydrostatic pressures applied to the sample.
4.3. Calculation of Ratios between Mean Square Relative Fluctuations of the Distribution Function Parameters and the Parameters Themselves

Table 8.
Square roots of mean-square relative fluctuations of the distribution function parameters divided by the according parameters (in percent).

Table 9.
Square roots of mean-square relative fluctuations of the distribution function parameters divided by the according parameters (in percent).
It was shown earlier [3] that if the activation barrier is represented as a set of ideal quantum oscillators then all the three parameters ν, m and νa have equal effect on the large thermodynamic fluctuation, since their appropriate ratios are all about 93%. As the data of Table 8 and Table 9 suggest, the situation is different for non-ideal quantum oscillators set. The model parameters do not influence the thermodynamic fluctuation to the same extent.
Particular attention should be given to the degeneracies Ω1 and Ω2. Their minor change of several percent will give rise to the large thermodynamic fluctuation responsible for unimolecular reaction progress.
5. Conclusions
Molecular tunneling is one of the most interesting and yet little-known low-temperature chemical processes. This is mainly because a tunneling molecule mass is three orders of magnitude greater than that of electron, so its de Broglie wavelength is small compared to a chemical bond length. Since this process occurs widely in nature, its investigation is a fundamental problem. The proposed interaction model of thermal equilibrium radiation with a molecule at low temperatures describes the process by using a distribution function. The function represents an activation barrier as the molecule’s sub-conformation states set. All the states share a common trait, that of providing the tunneling process by means of a part of zero-point vibrations energy.
To study the molecular tunneling process in more detail a concept of information entropy for activation process has been used. Its physical meaning was determined earlier [2]. The results of this investigation can be summarized as follows:
- The formula for information entropy of molecular tunneling has been derived.
- Arrhenius dependence for molecular tunneling process is caused by information entropy change. In addition, information entropies for low-temperature plateau and for the beginning of the activation part of Arrhenius dependence are equal.
- Probability of occurrence of the double proton transfer in the benzoic acid dimer has been calculated for sequential deuteration of the hydrogen bond and various hydrostatic pressures.
- Efficiency comparison has been made for Fe-CO bond recombination in β-hemoglobin and double proton transfer in benzoic acid dimer. The first reaction is more effective at low temperatures, while the temperature dependence of the second one is the opposite.
- There exists a correlation between mean-square fluctuations of the effective activation frequency and quantum oscillator frequency.
- The calculated mean-square fluctuations of the distribution function parameters indicate they do not influence a large entropy fluctuation to the same extent. Here the activation barrier is represented as a set of non-ideal quantum oscillators where the degeneracy and exclusion of some exited vibration levels are taken into account.
Author Contributions
The activation process model was created and developed by Anatoly V. Stepanov. He also did the numerical simulations. Maxim A. Stepanov derived the equations and wrote the paper.
Acknowledgments
We thank N.D. Knyazhevich and A.G. Svetashev for many helpful suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tavgin, V.L.; Stepanov, A.V. Activation Process Model. Phys. Status Solidi B 1990, 161, 123–130. [Google Scholar] [CrossRef]
- Stepanov, A.V. Interaction model of thermal radiation with molecule at low temperatures: Molecular tunnelling. J. Mol. Struct. 2002, 578, 47–61. [Google Scholar] [CrossRef]
- Stepanov, A.V. Information entropy of activation process: Application for low-temperature fluctuations of a myoglobin molecule. Int. J. Mod. Phys. B 2015, 29, 1550016. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Stepanov, A.V.; Tsvirko, M.P. Simulation of the double proton transfer dynamics in the benzoic acid dimer. J. Appl. Spectrosc. 2004, 71, 462–468. [Google Scholar] [CrossRef]
- Stepanov, A.V. Activation process model: Einstein coefficients for activation barrier. J. Mol. Struct. 2007, 805, 87–90. [Google Scholar] [CrossRef]
- Stepanov, A.V.; Tavgin, V.L. Development of the activation process model: Compensation effect. Int. J. Quantum Chem. 1996, 59, 7–14. [Google Scholar] [CrossRef]
- Stepanov, A.V. Modeling of metamaterials: A globular protein as a metamaterial prototype for electromagnetic-acoustic conversion at low temperatures. Proc. SPIE 2011, 8070. [Google Scholar] [CrossRef]
- Eiseinstein, L. Molecular tunneling in heme proteins. Int. J. Quantum Chem. 1976, 10, 21–27. [Google Scholar] [CrossRef]
- Stöckli, A.; Meier, B.H.; Kreis, R.; Meyer, R.; Ernst, R.R. Hydrogen bond dynamics in isotopically substituted benzoic acid dimers. J. Chem. Phys. 1990, 93, 1502–1520. [Google Scholar] [CrossRef]
- Horsewill, A.J.; McDonald, P.J.; Vijayaraghavan, D. Hydrogen bond dynamics in benzoic acid dimers as a function of hydrostatic pressure measured by nuclear magnetic resonance. J. Chem. Phys. 1994, 100, 1889–1894. [Google Scholar] [CrossRef]
- Einstein, A. Quantentheorie des einatomigen idealen Gases. Zweite Abhandlung. Sitzung Phys.-Math. Kl. 1925, 8, 3–14. [Google Scholar]
- Lesne, A. Shannon entropy: A rigorous notion at the crossroads between probability, information theory, dynamical systems and statistical physics. Math. Struct. Comput. Sci. 2014, 24, e240311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).