Synergistic Effect of YK-4-279 and Paclitaxel on A549 Cell Line †
Abstract
:1. Introduction
2. Materials and Methods
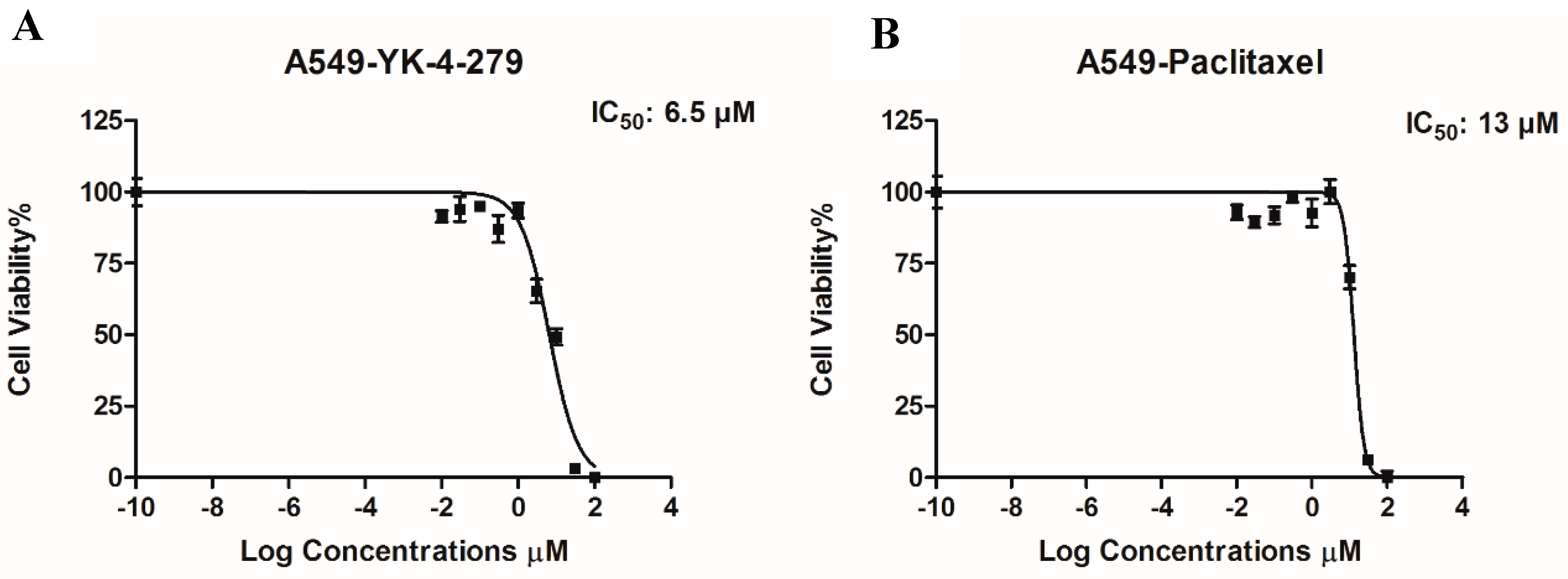
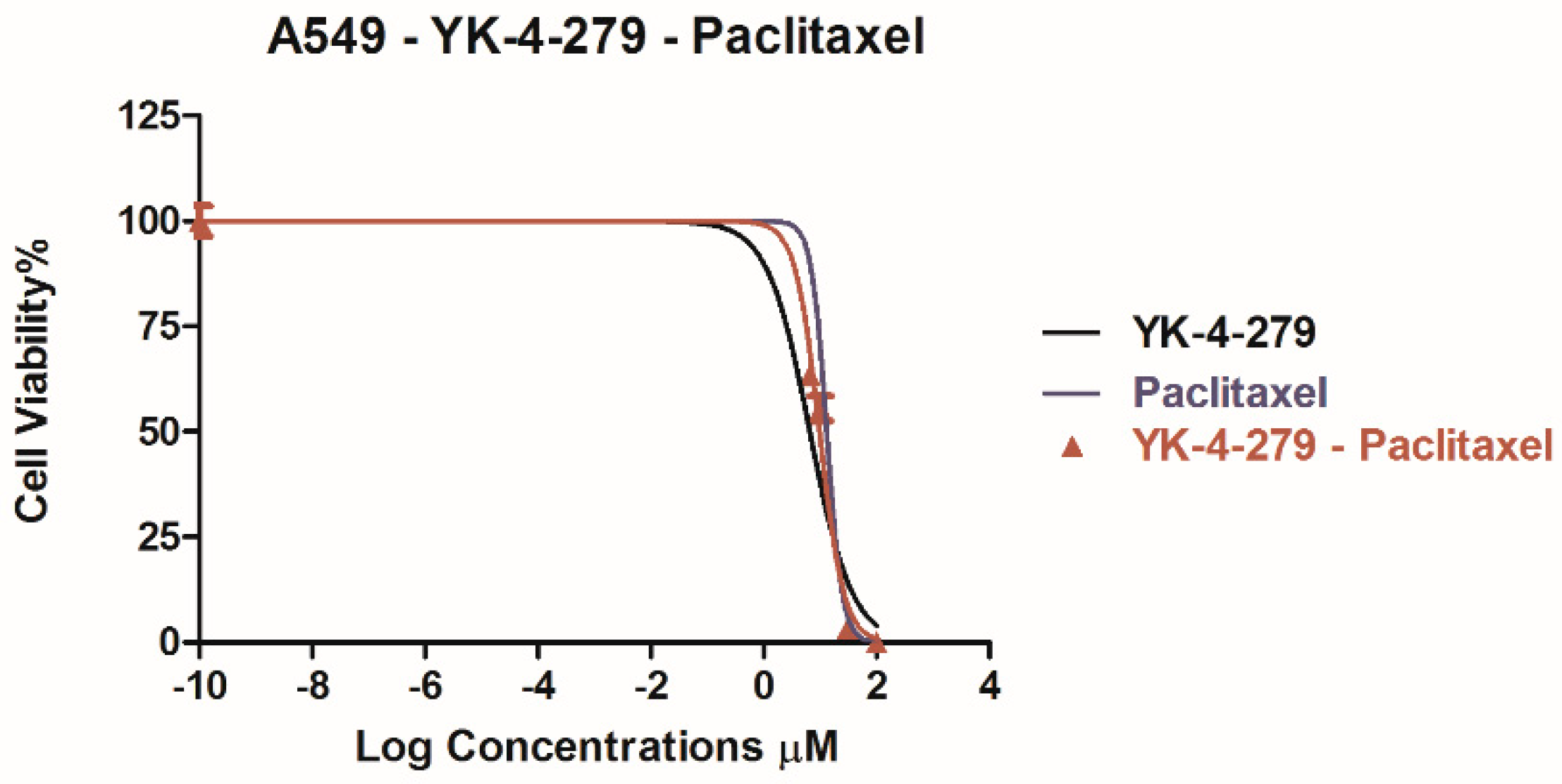
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncogenesis 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, H.; Ren, G. Epithelial-mesenchymal transition and drug resistance in breast cancer. Int. J. Oncol. 2015, 47, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bueno, G.; Portillo, F.; Cano, A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Watson, D.K. ETS transcription factors and their emerging roles in human cancer. Eur. J. Cancer 2005, 41, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.; Beauchamp, E.M.; Kong, Y.; Brown, M.L.; Toretsky, J.A.; Uren, A. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PLoS ONE 2011, 6, e19343. [Google Scholar] [CrossRef] [PubMed]
- Kollareddy, M.; Sherrard, A.; Park, J.H.; Szemes, M.; Gallacher, K.; Melegh, Z.; Oltean, S.; Michaelis, M.; Cinatl, J., Jr.; Kaidi, A.; et al. The small molecule inhibitor YK-4-279 disrupts mitotic progression of neuroblastoma cells, overcomes drug resistance and synergizes with inhibitors of mitosis. Cancer Lett. 2017, 403, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.W.; Huang, C.F.; Kaech, S.; Jacobson, C.; Banker, G.; Verhey, K.J. Posttranslational Modifications of Tubulin and the Polarized Transport of Kinesin-1 in Neurons. Mol. Biol. Cell 2010, 21, 572–583. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhatib, S.; Kandemiş, E.; Gizir, G.; Bulut, G. Synergistic Effect of YK-4-279 and Paclitaxel on A549 Cell Line. Proceedings 2018, 2, 1588. https://doi.org/10.3390/proceedings2251588
Alkhatib S, Kandemiş E, Gizir G, Bulut G. Synergistic Effect of YK-4-279 and Paclitaxel on A549 Cell Line. Proceedings. 2018; 2(25):1588. https://doi.org/10.3390/proceedings2251588
Chicago/Turabian StyleAlkhatib, Sara, Emine Kandemiş, Gamze Gizir, and Gülay Bulut. 2018. "Synergistic Effect of YK-4-279 and Paclitaxel on A549 Cell Line" Proceedings 2, no. 25: 1588. https://doi.org/10.3390/proceedings2251588
APA StyleAlkhatib, S., Kandemiş, E., Gizir, G., & Bulut, G. (2018). Synergistic Effect of YK-4-279 and Paclitaxel on A549 Cell Line. Proceedings, 2(25), 1588. https://doi.org/10.3390/proceedings2251588



