Combination of First Generation Proteasome Inhibitor Bortezomib with Temozolomide and Radiotherapy in Glioblastoma 2D and 3D Cell Cultures †
Abstract
:1. Introduction
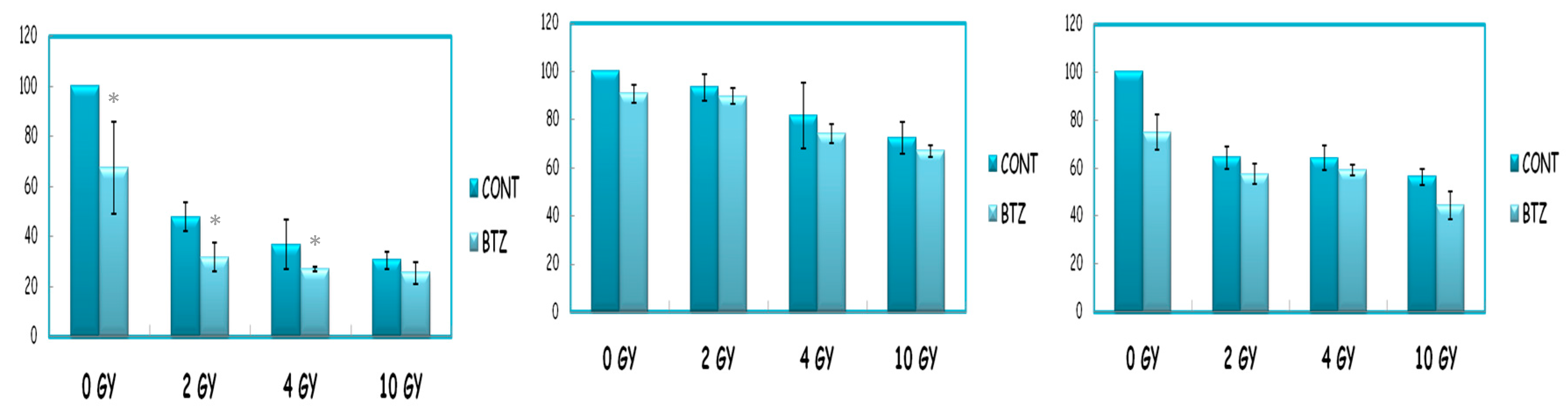
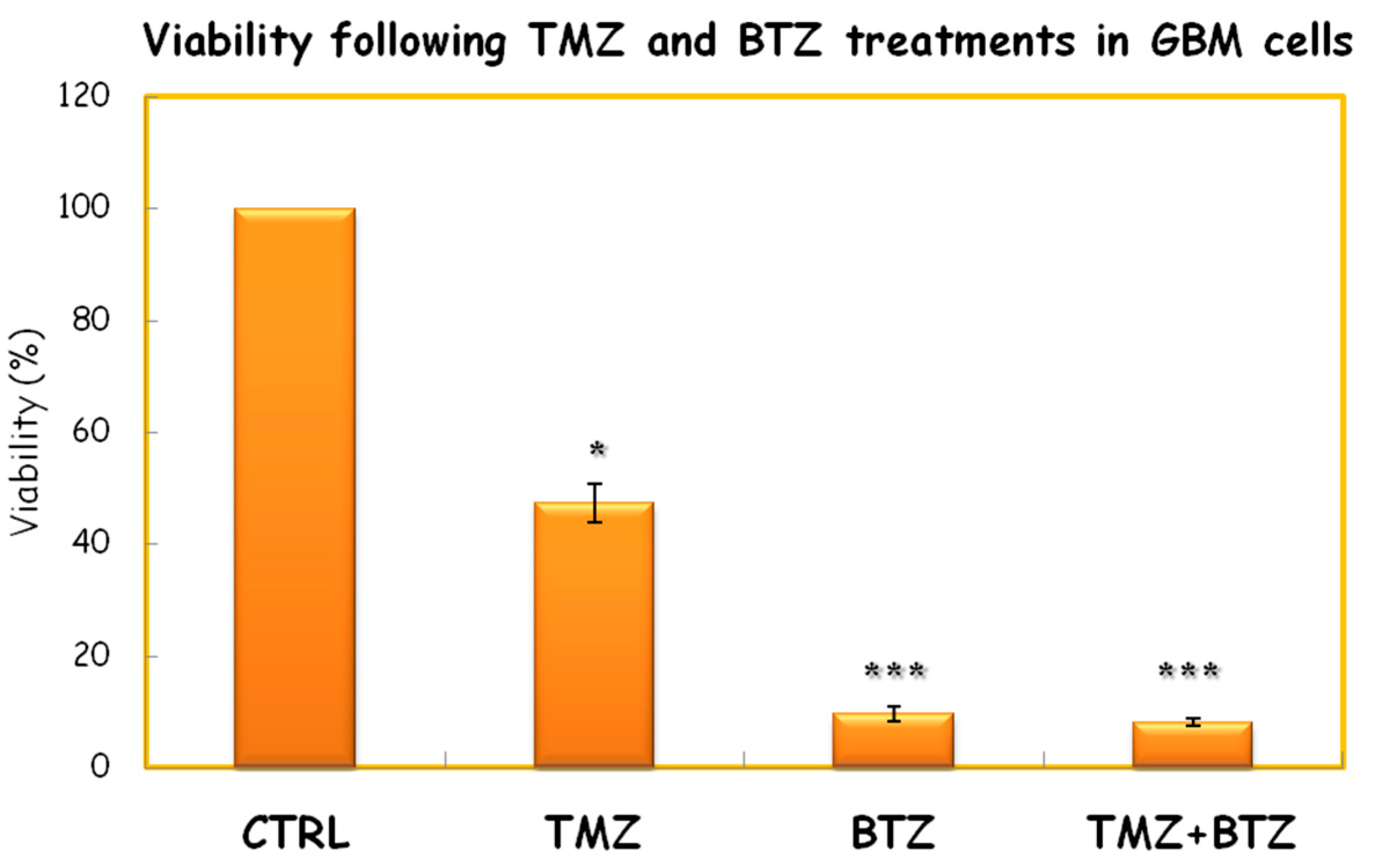
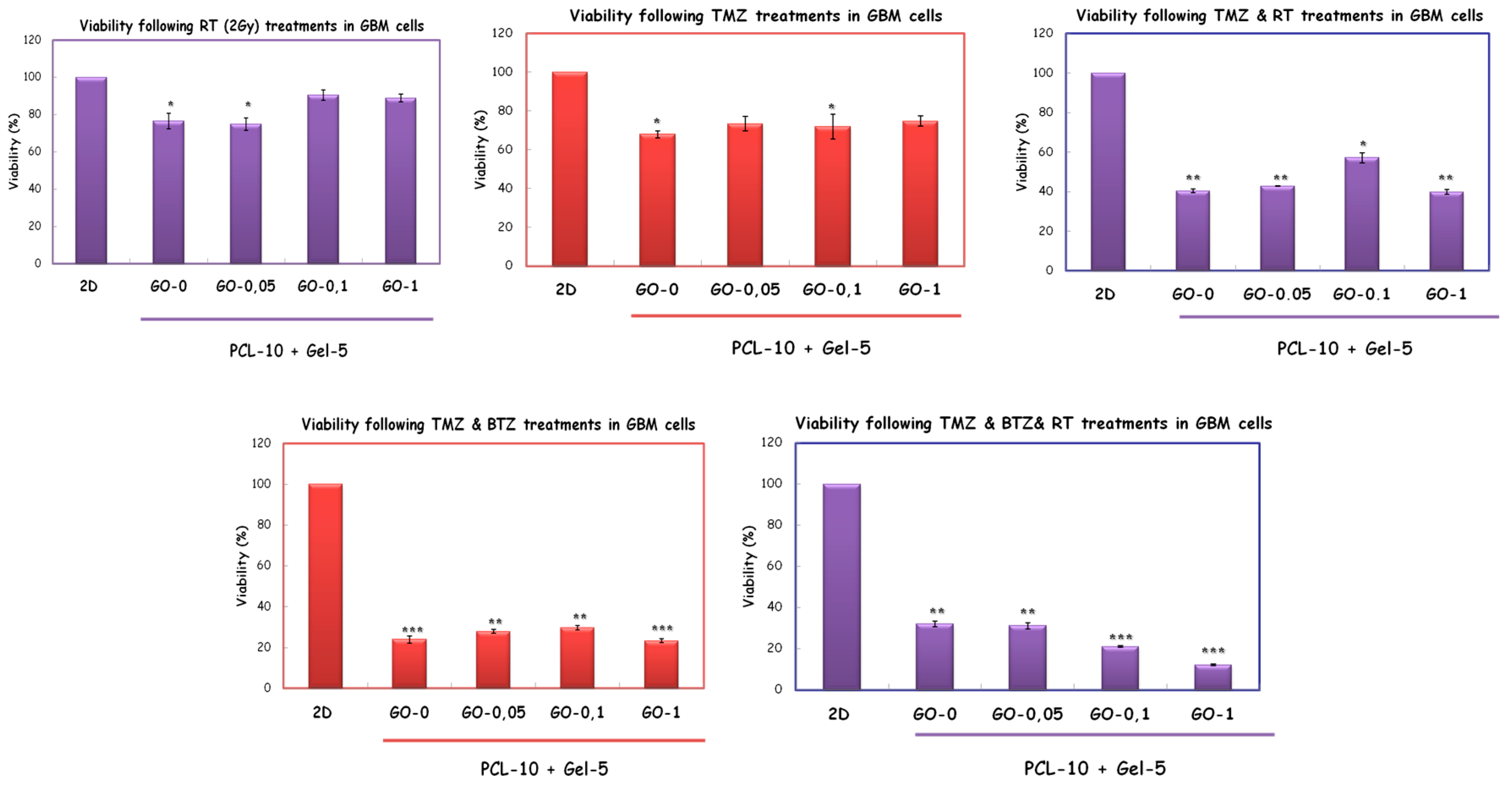
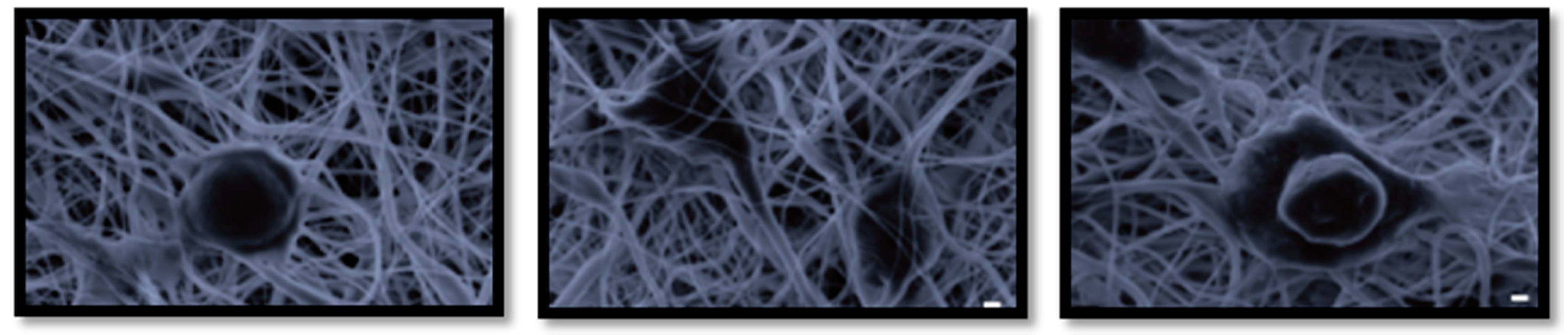
2. Materials and Methods
3. Discussion
Acknowledgments
References
- André-Grégoire, G.; Bidère, N.; Gavard, J. Temozolomide affects Extracellular Vesicles Released by Glioblastoma Cells. Biochimie 2018, 9084. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Hatzidaki, E.; Befani, C.D.; Liakos, P.; Papandreou, C.N. Bortezomib overcomes MGMT-related resistance of glioblastoma cell lines to temozolomide in a schedule-dependent manner. Investig. New Drugs 2013, 31, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Shafiei, S.S.; Asadi-Eydivand, M.; Ardeshir, M.; Solati-Hashjin, M. Graphene oxide- enriched poly (ε-caprolactone) electrospun nanocomposite scaffold for bone tissue engineering applications. J. Bioact. Compat. Polym. 2017, 32, 325–342. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Ding, G.; Xie, X.; Jiang, M. Preparation and characterization of graphene oxide/poly (vinyl alcohol) composite nanofibers via electrospinning. J. Appl. Polym. Sci. 2013, 127, 3026–3032. [Google Scholar] [CrossRef]
- Jalaja, K.; Sreehari, V.S.; Kumar, P.A.; Nirmala, R.J. Graphene oxide decorated electrospun gelatin nanofibers: Fabrication, properties and applications. Mater. Sci. Eng. C 2016, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.; Einsele, H.; Moreau, P.; San Miguel, J. Bortezomib, a novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treat. Rev. 2005, 31, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Nowicki, M.O.; Lee, C.H.; Chiocca, E.A.; Viapiano, M.S.; Lawler, S.E.; Lannutti, J.J. Quantitative analysis of complex glioma cell migration on electrospun polycaprolactone using time-lapse microscopy. Tissue Eng. Part C Methods 2009, 15, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Nelson, M.T.; Xue, R.; DeJesus, J.K.; Viapiano, M.S.; Lannutti, J.J.; Sarkar, A.; Winter, J.O. Mimicking white matter tract topography using core–shell electrospun nanofibers to examine migration of malignant brain tumors. Biomaterials 2013, 34, 5181–5190. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, S.; Unal, S.; Gokce, T.; Atasoy, B.M.; Gunduz, O.; Karademir, B. Combination of First Generation Proteasome Inhibitor Bortezomib with Temozolomide and Radiotherapy in Glioblastoma 2D and 3D Cell Cultures. Proceedings 2018, 2, 1579. https://doi.org/10.3390/proceedings2251579
Arslan S, Unal S, Gokce T, Atasoy BM, Gunduz O, Karademir B. Combination of First Generation Proteasome Inhibitor Bortezomib with Temozolomide and Radiotherapy in Glioblastoma 2D and 3D Cell Cultures. Proceedings. 2018; 2(25):1579. https://doi.org/10.3390/proceedings2251579
Chicago/Turabian StyleArslan, Sema, Semra Unal, Tilbe Gokce, Beste Melek Atasoy, Oguzhan Gunduz, and Betul Karademir. 2018. "Combination of First Generation Proteasome Inhibitor Bortezomib with Temozolomide and Radiotherapy in Glioblastoma 2D and 3D Cell Cultures" Proceedings 2, no. 25: 1579. https://doi.org/10.3390/proceedings2251579
APA StyleArslan, S., Unal, S., Gokce, T., Atasoy, B. M., Gunduz, O., & Karademir, B. (2018). Combination of First Generation Proteasome Inhibitor Bortezomib with Temozolomide and Radiotherapy in Glioblastoma 2D and 3D Cell Cultures. Proceedings, 2(25), 1579. https://doi.org/10.3390/proceedings2251579





