1. Introduction
Apoptosis, a programmed cell death, has a vital role in various cellular processes. Abnormal rate of apoptosis is closely associated with the pathophysiology of many diseases including cancer, autoimmune disorders and neurodegenerative disorders [
1]. Apoptotic cells exhibits morphological and biochemical changes; at most lipids, proteins and DNA molecules are significantly perturbed. A change in the plasma membrane is one of the earliest responses to apoptosis, caused by translocation of phosphatidylserine from the inner to the outer leaflet of lipid bilayer. Existence of changes in proteins (e.g., caspase activation) and DNA fragmentation are other hallmarks of apoptosis [
1,
2].
Detection of apoptosis involves a variety of biochemical assays (caspase assays, mitochondrial dyes, DNA laddering technique) and flow cytometric analysis [
1]. Flow cytometry is a powerful tool for analysis of apoptotic cell death. In this system, thousands of cells are detected by the laser beam as they pass in front of the laser and fluorescence-stained cells are stimulated by laser light. The signals obtained allow collection of information about the cell size, complexity and molecules associated with cell-bound antibodies [
3]. Recently, Fourier transform infrared (FTIR) spectroscopy has been extensively used for analysis of biological samples [
4,
5,
6,
7]. FTIR spectroscopy detects the vibrational modes of functional groups of all cellular macromolecules and able to sense cellular changes like plasma membrane perturbations (ordering/disordering status, membrane fluidity), protein secondary structural changes, DNA/RNA and metabolic changes. Thus, it can be alternatively used for detection of apoptosis. The aim of this study was to detect early apoptosis in human Jurkat T lymphocytes treated with cytotoxic camptothecin agent that induces apoptosis by using both FTIR spectroscopy and flow cytometry. The results obtained from both techniques are well matched that Jurkat T cells underwent early apoptosis, exhibiting changes in the plasma membrane phospholipids and proteins.
2. Materials and Methods
Jurkat T cells were grown in ATCC-formulated RPMI-1640 medium with fetal bovine serum to a final concentration of 10%. Cultures were maintained at a cell concentration between 1 × 105 and 1 × 106 viable cells/mL. Cells were grown to reach to adequate cell numbers. After washing and counting procedures of the cells, they were incubated with 4 µM Camptothecin (CPT) in RPMI (with 5% FCS) for 24 h. Immunoflourescence labeling of Jurkat cells for multicolor flow cytometry was carried out with Annexin V concomitantly with 7-AAD. The same cells were resuspended in the NaCl solution and measured with an IRTracer-100 FTIR spectrometer (Shimadzu, Japan) combined with an attenuated total reflection (ATR) unit and equipped with a DLATGS detector. A 1.8 μL amount of cell (106 cell/mL) was deposited on the ATR plate and dried prior to measurements.
3. Results
The presence of inner-membrane phosphatidylserine (PS) on the outer membrane of a cell is one of the earliest events in apoptosis. This process can be monitored by Annexin V, which binds to negatively charged PS, in conjunction with 7AAD that has strong affinity for DNA and is efficiently excluded by intact cells. CPT-treated Jurkat cells were mostly in Annexin V+/7AAD-phenotype compared to the negative controls. This reflects an early apoptotic changes in cells detected by flow cytometry. Additionally, the plasma membrane did not lose its integrity since these cells displayed a 7-AAD negative profile.
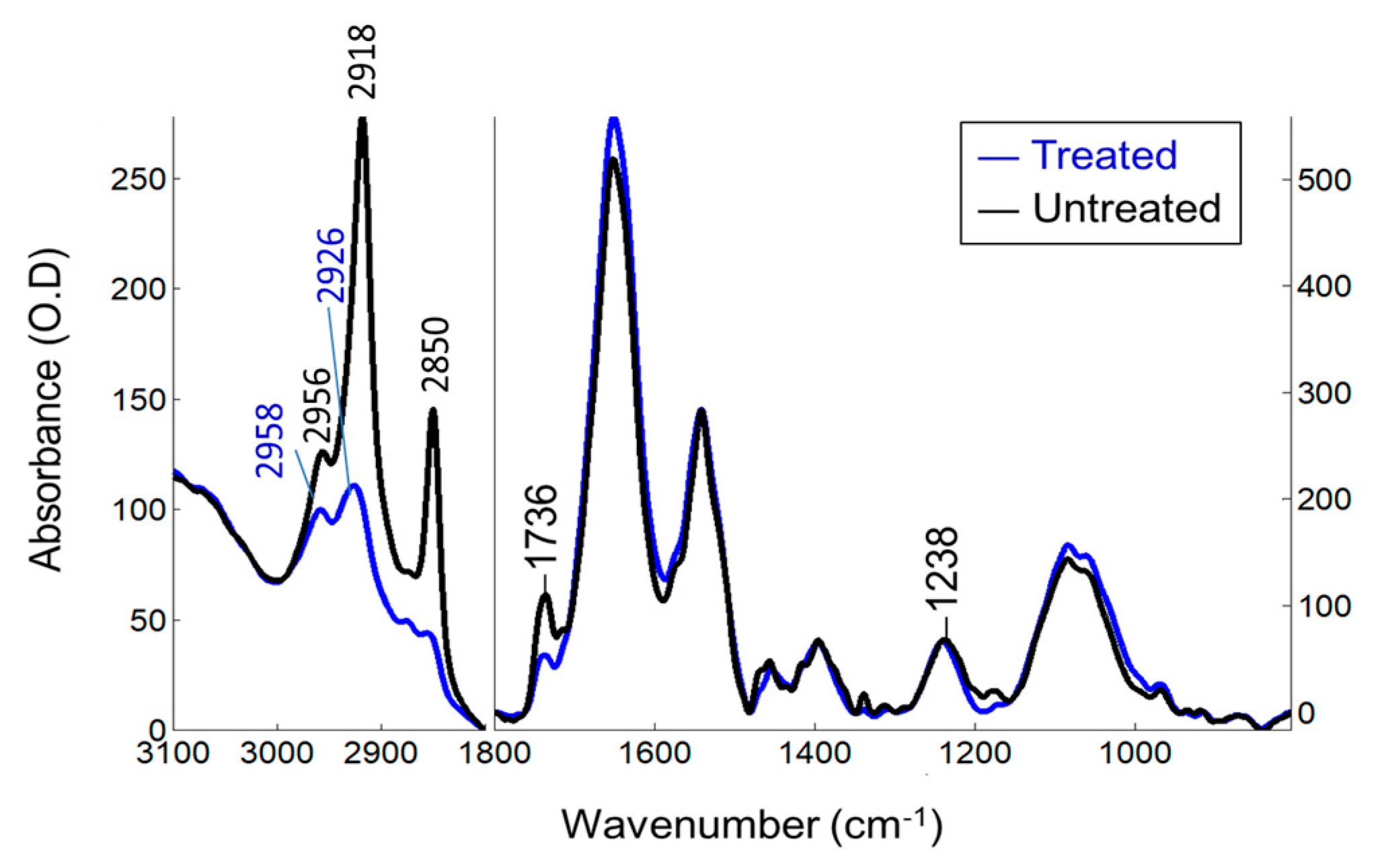
The FTIR mean spectra of the treated and untreated Jurkat T cells (
Figure 1) comprise mainly of lipids (3000–2800 cm
−1), proteins (1700–1600 cm
−1), nucleic acids (~1230, ~1080 cm
−1) and carbohydrates (below 1190 cm
−1) (for band assignments see [
4,
5]). When compared to untreated Jurkat T cells, CPT-treated cells exhibit a combined decrease in the lipid signals due to the cell membrane phospholipids. The intensity of the CH
2 and CH
3 signals (3000–2800 cm
−1) decreased, broadened and upshifted, indicating that fatty acyl chains of membrane phospholipids are disordered and becomes more mobile after CPT-treatment. The peak intensities at 1736 cm
−1 due to the lipid ester C=O stretching and at 1238 cm
−1 due to the phosphate groups diminished and their band position upshifted, indicating alterations in their H-bonding properties and microenvironment. Herein, interpretation on DNA is not unambiguous since their phosphate peaks are superimposed with phospholipids. The protein structures are also influenced by treatment (1700–1600 cm
−1).
4. Discussion
Flow cytometry data revealed that CPT-treated Jurkat T cells were Annexin V positive and 7AAD negative, indicating early cell death stage. Only phosphatidylserine is present on the surface of the plasma membrane; but the plasma membrane is not disturbed yet, indicative of induction of apoptosis in the CPT-treated T cells. Based on the FTIR data, Jurkat T cell membrane phospholipids underwent dynamical and structural changes after treatment. Hydrophobic packing of tails, lipid esters in the interfacial region and hydrophilic phosphate groups of plasma membrane are significantly affected. Observation of both protein and lipid changes is most likely associated with the plasma membrane alterations, which might result from the early apoptosis process after CPT-treatment.
In conclusion, a combined study of flow cytometry and FTIR spectroscopy for the analysis of apoptosis in human T cells exhibited compatible and complementary results. Both techniques could successfully detect biochemical and biophysical features of T cells.