Evaluation of Zeolite Adsorption Properties for Cu(II) Removal from Acidic Aqueous Solutions in Fixed-Bed Column System †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Adsorbate
2.3. Instrumentation
- A Colorimeter DR890 (HACH LANGE, Düsseldorf, Germany) with appropriate reagent was used to determine amount of dissolved Cu(II).
- pH values were determined according to pH meter inoLab ph 730 (WTW, Germany) which was standardized using buffer solutions of different pH values (4.01, 7.00)
2.4. Column System
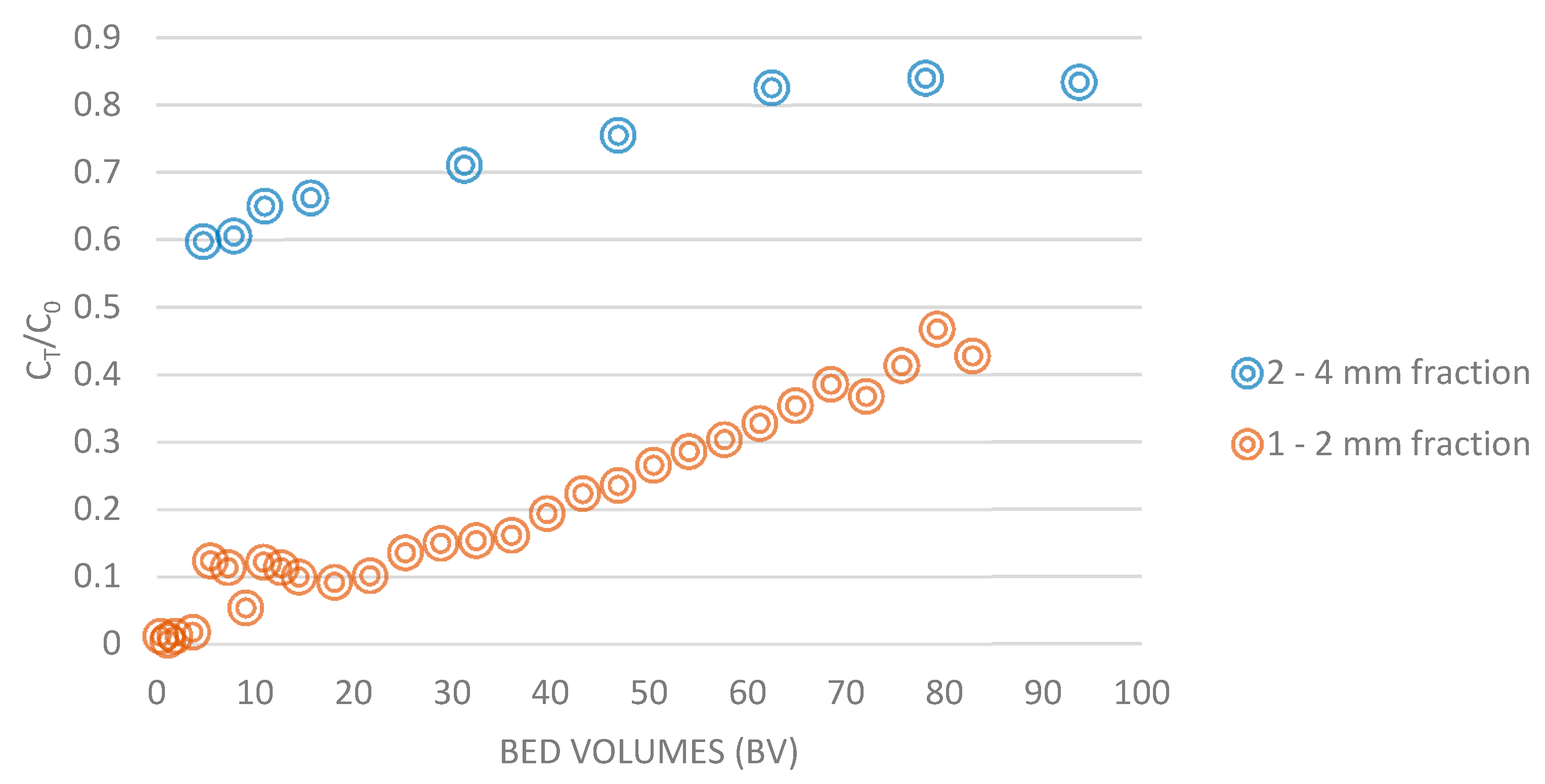
3. Results and Discussion
Column System
4. Conclusions
Acknowledgments
References
- Dstan, L.; DehghaniI, M. Removal of cadmium from industrial waste water by adsorption zeolite clinoptilolite. Biosci. Biotechnol. Res. Commun. 2016, 9, 865–871. [Google Scholar] [CrossRef]
- Wirth, P.; Harfst, J. Challenges of Post-Mining Regions in Central Europe; IÖR: München, Germany, 2012. [Google Scholar]
- Kupka, D.; Pállová, Z.; Horňáková, A.; Achimovičová, M.; Kavečanský, V. Effluent water quality and the ochre deposit characteristics of the abandoned Smolnik mine, East Slovakia. Acta Montan. Slov. 2012, 17, 56. [Google Scholar]
- Ibrahimi, M.M.; Sayyadi, A.S. Application of natural and modified zeolites in removing heavy metal cations from aqueous media: An overview of including parameters affecting the process. Int. J. Geol. Agric. Environ. Sci. 2015, 3, 1–7. [Google Scholar]
- Michalev, T.; Petrov, I. The Removal of Heavy Metal Ions by Synthetic Zeolites: A Review. Proc. Univ. Ruse 2012, 51, 79–84. [Google Scholar]
- Jovanovic, M.; Arcon, I.; Kovac, J.; Tusar, N.N.; Obradovic, B.; Rajic, N. Removal of manganese in batch and fluidized bed systems using beads of zeolite as adsorbent. Microporous Mesoporous Mater. 2016, 226, 378–385. [Google Scholar] [CrossRef]
- Holub, M.; Balintova, M.; Pavlikova, P.; Palascakova, L. Study of sorption properties of zeolite in acidic conditions in dependence on particle size. Chem. Eng. Trans. 2013, 32, 559–564. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Biškup, B.; Subotić, B. Kinetic analysis of the exchange processes between sodium ions from zeolite A and cadmium, copper and nickel ions from solutions. Sep. Purif. Technol. 2004, 37, 17–31. [Google Scholar] [CrossRef]
- Mudasir, M.; Karelius, K.; Aprilita, N.H.; Wahyuni, E.T. Adsorption of mercury (II) on dithizone-immobilized natural zeolite. J. Environ. Chem. Eng. 2016, 4, 1839–1849. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Medvidović, N.V. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Massadeh, A.M.; Younes, H.A. Natural Jordanian zeolite: Removal of heavy metal ions from water samples using column and batch method. Environ. Monit. Assess. 2009, 157, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, M.A.; Hadjiconstantinou, M.P.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Use of natural clinoptilolite for the removal of lead, copper and zinc in fixed bed column. J. Hazard. Mater. 2007, 143, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, F. Ion Exchange; Dover Publications Inc.: New York, NY, USA, 1995. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holub, M.; Balintova, M.; Kovacova, Z. Evaluation of Zeolite Adsorption Properties for Cu(II) Removal from Acidic Aqueous Solutions in Fixed-Bed Column System. Proceedings 2018, 2, 1293. https://doi.org/10.3390/proceedings2201293
Holub M, Balintova M, Kovacova Z. Evaluation of Zeolite Adsorption Properties for Cu(II) Removal from Acidic Aqueous Solutions in Fixed-Bed Column System. Proceedings. 2018; 2(20):1293. https://doi.org/10.3390/proceedings2201293
Chicago/Turabian StyleHolub, Marian, Magdalena Balintova, and Zdenka Kovacova. 2018. "Evaluation of Zeolite Adsorption Properties for Cu(II) Removal from Acidic Aqueous Solutions in Fixed-Bed Column System" Proceedings 2, no. 20: 1293. https://doi.org/10.3390/proceedings2201293
APA StyleHolub, M., Balintova, M., & Kovacova, Z. (2018). Evaluation of Zeolite Adsorption Properties for Cu(II) Removal from Acidic Aqueous Solutions in Fixed-Bed Column System. Proceedings, 2(20), 1293. https://doi.org/10.3390/proceedings2201293





