Preparation and Identification of BaFe2O4 Nanoparticles by the Sol–Gel Route and Investigation of Its Microwave Absorption Characteristics at Ku-Band Frequency Using Silicone Rubber Medium †
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Synthesis of BaFe2O4 Nanoparticles
2.3. Preparation of BaFe2O4/Silicone Rubber Nanocomposite
3. Results and Discussion
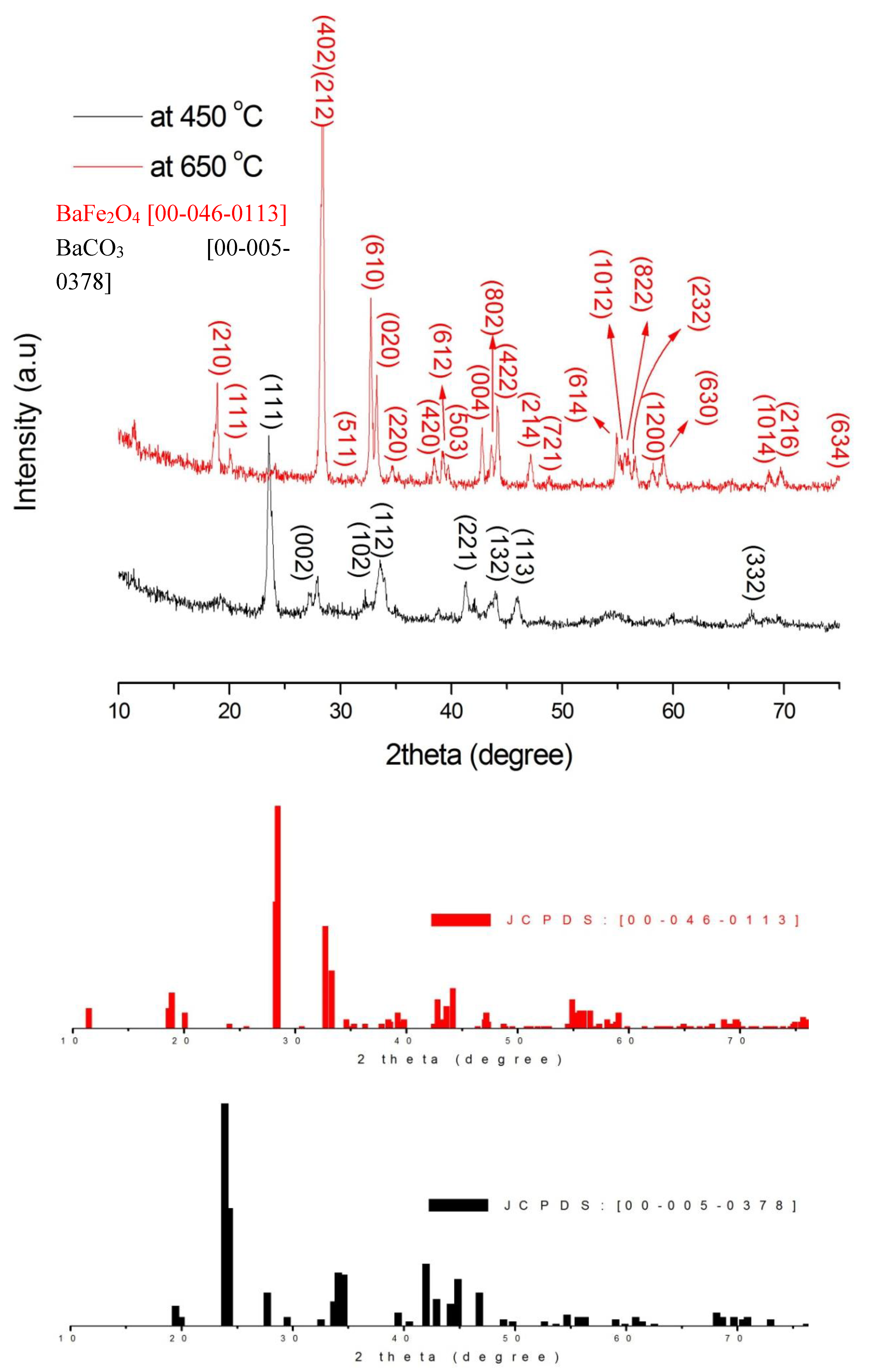
3.1. Phase Identification Analysis
3.2. FE-SEM Morphology
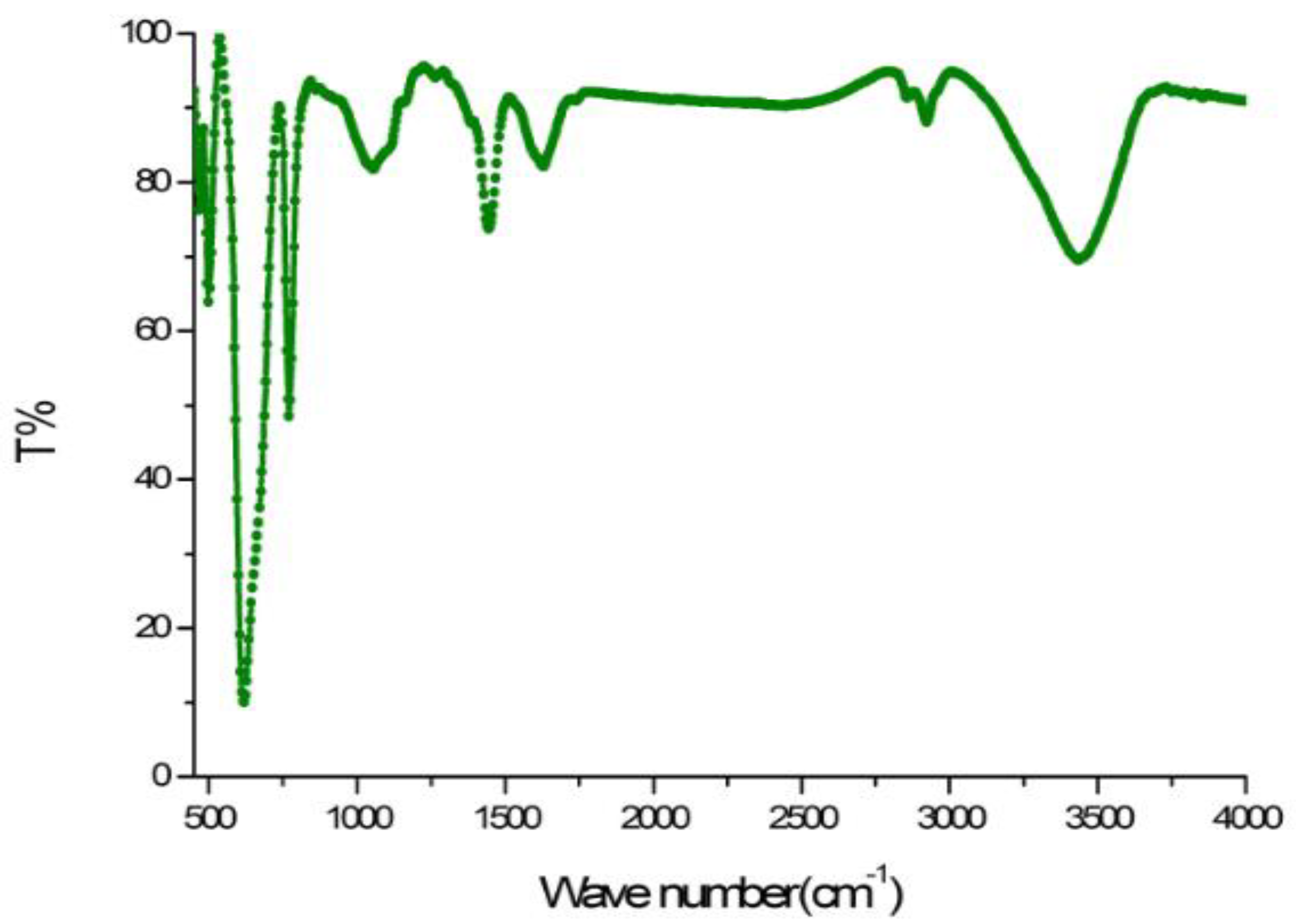
3.3. FTIR Spectroscopy
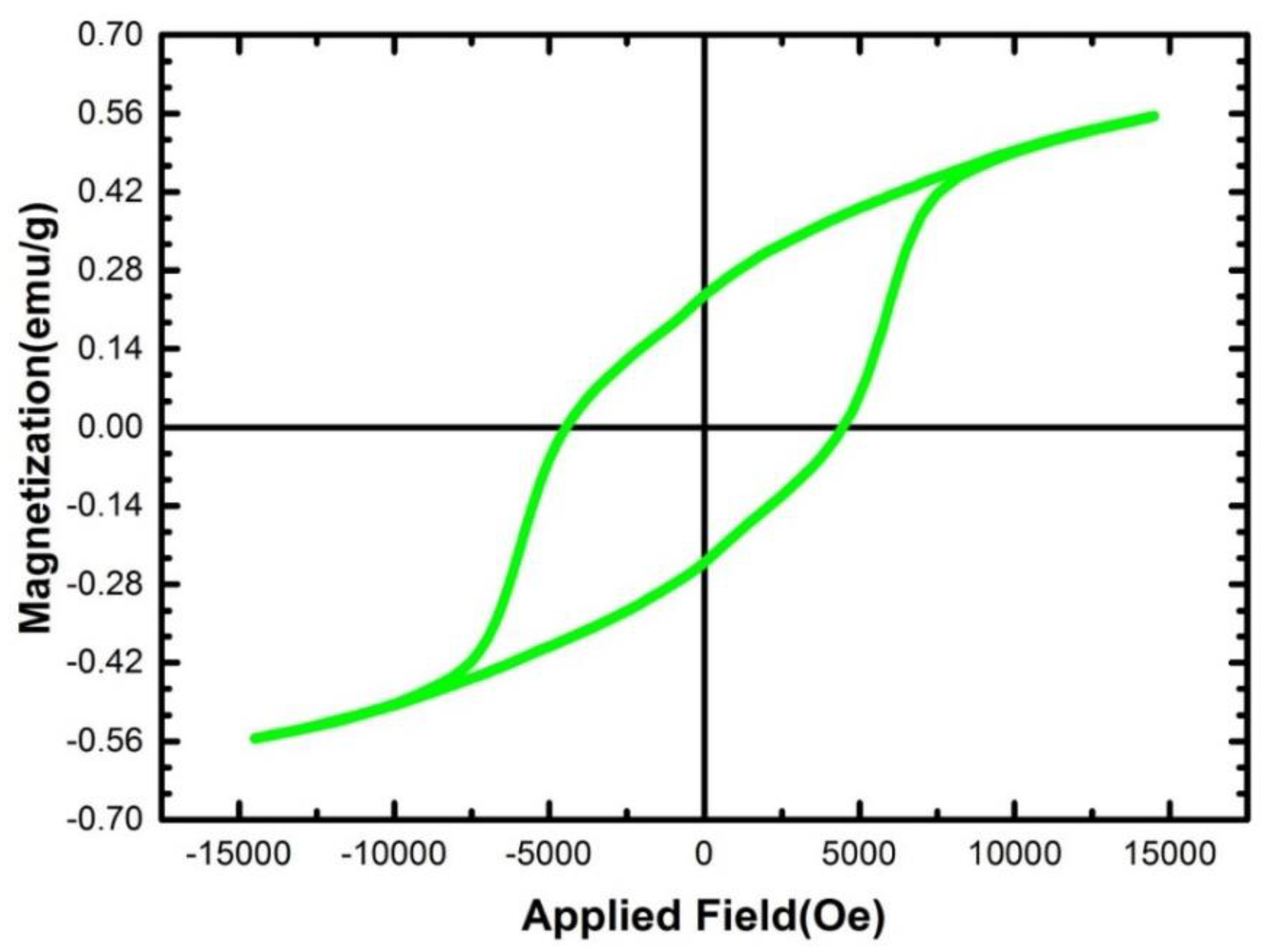
3.4. Magnetic Properties
3.5. Microwave Absorption Properties
4. Conclusions
References
- Galvão, W.S.; Neto, D.; Freire, R.M.; Fechine, P. Super-paramagnetic nanoparticles with spinel structure: A review of synthesis and biomedical applications. In Solid State Phenomena; Trans Tech Publications: Zürich, Switzerland, 2016; pp. 139–176. [Google Scholar]
- Mandizadeh, S.; Salavati-Niasari, M.; Sadri, M. Hydrothermal synthesis, characterization and magnetic properties of BaFe2O4 nanostructure as a photocatalytic oxidative desulfurization of dibenzothiophene. Sep. Purif. Technol. 2017, 175, 399–405. [Google Scholar] [CrossRef]
- Lemine, O.; Bououdina, M.; Sajieddine, M.; Al-Saie, A.; Shafi, M.; Khatab, A.; Al-Hilali, M.; Henini, M. Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Phys. B Condens. Matter 2011, 406, 1989–1994. [Google Scholar] [CrossRef]
- Peymanfar, R.; Javanshir, S. Preparation and characterization of Ba0.2Sr0.2La0.6MnO3 nanoparticles and investigation of size & shape effect on microwave absorption. J. Magn. Magn. Mater. 2017, 432, 444–449. [Google Scholar]
- Saravani, H.; Esmaeilzaei, M.R.; Ghahfarokhi, M.T. Synthesis and Characterization of Ferromagnetic BaFe2 O4 Nanocrystals Using Novel Ionic Precursor Complex [Fe(opd)3]2[Ba(CN)8]. J. Inorg. Organomet. Polym. Mater. 2016, 26, 353–358. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Xu, D.; Zhang, L. Microwave-Assisted Catalytic Degradation of Crystal Violet with Barium Ferrite Nanomaterial. Ind. Eng. Chem. Res. 2016, 55, 11869–11877. [Google Scholar] [CrossRef]
- Shen, P.; Luo, J.; Zuo, Y.; Yan, Z.; Zhang, K. Effect of La-Ni substitution on structural, magnetic and microwave absorption properties of barium ferrite. Ceram. Int. 2017, 43, 4846–4851. [Google Scholar] [CrossRef]
- Ali, K.; Iqbal, J.; Jana, T.; Ahmad, N.; Ahmad, I.; Wan, D. Enhancement of microwaves absorption properties of CuFe2O4 magnetic nanoparticles embedded in MgO matrix. J. Alloys Compd. 2017, 696, 711–717. [Google Scholar] [CrossRef]
- Bahadur, A.; Saeed, A.; Iqbal, S.; Shoaib, M.; Ahmad, I.; ur Rahman, M.S.; Bashir, M.I.; Yaseen, M.; Hussain, W. Morphological and magnetic properties of BaFe12O19 nanoferrite: A promising microwave absorbing material. Ceram. Int. 2017, 43, 7346–7350. [Google Scholar] [CrossRef]
- Wang, M.; An, K.; Fang, Y.; Wei, G.; Yang, J.; Sheng, L.; Yu, L.; Zhao, X. The microwave absorbing properties of CoFe2 attached single walled carbon nanotube/BaFe12O19 nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 12475–12483. [Google Scholar] [CrossRef]
- Afzali, A.; Mottaghitalab, V.; Afghahi, S.S.; Jafarian, M. The coating of composite nanoparticles of BaFe12O19/multi-walled carbon nanotubes using silicon matrix on nonwoven substrate for radar absorption in X and Ku bands. J. Ind. Text. 2018, 47, 1867–1886. [Google Scholar] [CrossRef]
- Feng, H.; Bai, D.; Tan, L.; Chen, N.; Wang, Y. Preparation and microwave-absorbing property of EP/BaFe12O19/PANI composites. J. Magn. Magn. Mater. 2017, 433, 1–7. [Google Scholar] [CrossRef]
- Afghahi, S.S.S.; Peymanfar, R.; Javanshir, S.; Atassi, Y.; Jafarian, M. Synthesis, characterization and microwave characteristics of ternary nanocomposite of MWCNTs/doped Sr-hexaferrite/PANI. J. Magn. Magn. Mater. 2017, 423, 152–157. [Google Scholar] [CrossRef]
- Peymanfar, R.; Javidan, A.; Javanshir, S. Preparation and investigation of structural, magnetic, and microwave absorption properties of aluminum-doped strontium ferrite/MWCNT/polyaniline nanocomposite at KU-band frequency. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ma, Z.; Mang, C.; Weng, X.; Zhang, Q.; Si, L.; Zhao, H. The Influence of Different Metal Ions on the Absorption Properties of Nano-Nickel Zinc Ferrite. Materials 2018, 11, 590. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Yang, J.; Luo, H.; Chen, F.; Wang, X.; Gong, R. Enhanced Microwave Absorption and Surface Wave Attenuation Properties of Co0.5Ni0.5Fe2O4 Fibers/Reduced Graphene Oxide Composites. Materials 2018, 11, 508. [Google Scholar] [CrossRef]
- Peymanfar, R.; Javanshir, S.; Naimi-Jamal, M.R. Preparation and characterization of MWCNT/Zn0.25Co0.75Fe2O4 nanocomposite and investigation of its microwave absorption properties at x-band by silicone rubber polymeric matrix. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, Santiago, Spain, 1–30 November 2017. [Google Scholar]
- Seyed, A.S.S.; Peymanfar, R.; Javanshir, S.; Javidan, A. Preparation and investigation of structural, magnetic and microwave absorption properties of Ba0.2Sr0.2La0.6MnO3/MWCNT nanocomposite in comparison with Ba0.2Sr0.2La0.6MnO3 in x-band region. Nanoscale 2015, 2, 73–80. [Google Scholar]
- Cao, W.-Q.; Wang, X.-X.; Yuan, J.; Wang, W.-Z.; Cao, M.-S. Temperature dependent microwave absorption of ultrathin graphene composites. J. Mater. Chem. C 2015, 3, 10017–10022. [Google Scholar] [CrossRef]
- Dalal, M.; Greneche, J.-M.; Satpati, B.; Ghzaiel, T.B.; Mazaleyrat, F.; Ningthoujam, R.S.; Chakrabarti, P.K. Microwave Absorption and the Magnetic Hyperthermia Applications of Li0.3Zn0.3Co0.1Fe2.3O4 Nanoparticles in Multiwalled Carbon Nanotube Matrix. ACS Appl. Mater. Interfaces 2017, 9, 40831–40845. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Dai, J.; Wang, L.; Yang, H. Synthesis and microwave absorption properties of plate-like BaFe12O19@Fe3O4 core-shell composite. J. Alloys Compd. 2018, 739, 202–210. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Hou, Y.; Li, J.; Li, L. Synthesis and microwave absorption properties of coiled carbon nanotubes/CoFe2O4 composites. Ceram. Int. 2016, 42, 17814–17821. [Google Scholar] [CrossRef]
- Yang, H.; Ye, T.; Lin, Y.; Liu, M. Preparation and microwave absorption property of graphene/BaFe12O19/CoFe2O4 nanocomposite. Appl. Surface Sci. 2015, 357, 1289–1293. [Google Scholar] [CrossRef]
- Tang, X.; Wei, G.; Zhu, T.; Sheng, L.; An, K.; Yu, L.; Liu, Y.; Zhao, X. Microwave absorption performance enhanced by high-crystalline graphene and BaFe12O19 nanocomposites. J. Appl. Phys. 2016, 119, 204301. [Google Scholar] [CrossRef]
- Nikmanesh, H.; Moradi, M.; Bordbar, G.H.; Alam, R.S. Synthesis of multi-walled carbon nanotube/doped barium hexaferrite nanocomposites: An investigation of structural, magnetic and microwave absorption properties. Ceram. Int. 2016, 42, 14342–14349. [Google Scholar] [CrossRef]
- Ezzati, S.N.; Rabbani, M.; Leblanc, R.M.; Asadi, E.; Ezzati, S.M.H.; Rahimi, R.; Azodi-Deilami, S. Conducting, magnetic polyaniline/Ba0.25Sr0.75Fe11(Ni0.5Mn0.5)O19 nanocomposite: Fabrication, characterization and application. J. Alloys Compd. 2015, 646, 1157–1164. [Google Scholar] [CrossRef]


| Particles | Max RL (dB) | Diameter (mm) | Absorption Bandwidth (GHz) < −10 dB | Ref. |
|---|---|---|---|---|
| BaFe12O19/Fe3O4 | 33.6 | 2.5 | 1.3 | [21] |
| CoFe2O4 | 14 | 3 | 2 | [22] |
| BaFe12O19/CoFe2O4 | 10 | 5 | - | [23] |
| BaFe12O19 | 16.1 | 3 | 3.8 | [24] |
| BaCu0.5Mg0.5ZrFe10O19 | 9 | 2.1 | - | [25] |
| BaFe12O19 | 7 | 2.5 | - | [11] |
| Ba0.25Sr0.75 Fe11(Ni0.5Mn0.5)O19 | 3.6 | 4 | - | [26] |
| BaFe12O19 | 10.7 | 3 | - | [12] |
| Ba0.2Sr0.2La0.6MnO3 | 22.36 | 2 | 2.67 | [4] |
| BaFe2O4 | 51.67 | 1.75 | <5.6 | Presented study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peymanfar, R.; Rahmanisaghieh, M.; Ghaffari, A.; Yassi, Y. Preparation and Identification of BaFe2O4 Nanoparticles by the Sol–Gel Route and Investigation of Its Microwave Absorption Characteristics at Ku-Band Frequency Using Silicone Rubber Medium. Proceedings 2018, 2, 5234. https://doi.org/10.3390/ecms2018-05234
Peymanfar R, Rahmanisaghieh M, Ghaffari A, Yassi Y. Preparation and Identification of BaFe2O4 Nanoparticles by the Sol–Gel Route and Investigation of Its Microwave Absorption Characteristics at Ku-Band Frequency Using Silicone Rubber Medium. Proceedings. 2018; 2(17):5234. https://doi.org/10.3390/ecms2018-05234
Chicago/Turabian StylePeymanfar, Reza, Mitra Rahmanisaghieh, Arezoo Ghaffari, and Yousef Yassi. 2018. "Preparation and Identification of BaFe2O4 Nanoparticles by the Sol–Gel Route and Investigation of Its Microwave Absorption Characteristics at Ku-Band Frequency Using Silicone Rubber Medium" Proceedings 2, no. 17: 5234. https://doi.org/10.3390/ecms2018-05234
APA StylePeymanfar, R., Rahmanisaghieh, M., Ghaffari, A., & Yassi, Y. (2018). Preparation and Identification of BaFe2O4 Nanoparticles by the Sol–Gel Route and Investigation of Its Microwave Absorption Characteristics at Ku-Band Frequency Using Silicone Rubber Medium. Proceedings, 2(17), 5234. https://doi.org/10.3390/ecms2018-05234





