Abstract
Ammonia detection at low temperatures below 150 °C is attractive to be well suited for flexible substrates in terms of thermal strain and to specific environment not allowing high temperature such as explosive one. In commercial gas sensors, tungsten trioxide is the mostly used semiconducting metal oxide after tin dioxide. We report herein the efficiency of tungsten trioxide nanowires deposited on rigid substrate by drop coating from colloidal solution. This study provides an interesting approach to fabricate ammonia sensors on conformable substrate with significant properties for applications in environmental monitoring devices.
1. Introduction
Poor air quality is a global concern. Around us, ammonia (NH3) is a natural toxic and flammable gas present throughout the atmosphere. It is used in manufacturing of fertilizers, explosives, cleaning fluids and in pharmaceutical applications. It is a colorless gas with a characteristic pungent smell and it can affect environment and human health. The National Institute for Occupational Safety and Health (NIOSH) Recommended Exposure Limit (REL) for ammonia is 25 ppm (18 mg/m3) averaged over an eight-hour workday and a Short Term Exposure Limit (STEL) of 35 ppm (27 mg/m3) during any 15 min period in the day. Due to these reasons, ammonia monitoring has received considerable attention. Numerous methods have been developed and are in use for ammonia measurement [1]. Since 1962, it has been demonstrated that absorption or desorption of a gas on a metal oxide surface changes the material conductivity [2]. Then, Metal Oxide Semiconductors (MOS) have been applied increasingly as conventional chemical sensing materials because of their simplicity in measurement setup, their miniaturization facility for portable instruments, their high sensitivity and their relatively low cost [3]. Conductometric gas sensor based on WO3 sensing material is demonstrated to respond to NH3 for gas leak detection indoor and outdoor air quality monitoring [4]. Nevertheless, high working temperatures (275–375 °C) and recovery behaviors limit the widespread use of this material. However, when the active layers have a large surface area, it means a high surface/volume ratio, the surface adsorbs as much of the target gas as possible and gives a stronger and easily measurable electrical signal [5]. This work aims to investigate the gas-sensing performances of tungsten trioxide (WO3) nanowires (NWs) at low temperature.
2. Materials and Methods
Our conductometric gas sensor consists of Ti/Pt interdigitated electrodes (100 nm thick and 50 µm width, respectively), deposited on Si/SiO2 by magnetron radiofrequency sputtering and a sensitive WO3−x based NWs on the top of them. A heating source can be provided underneath if needed inside the chamber. Ultrathin WO3−x NWs were synthesized by a solvothermal method using slight modifications from our previous work [6]. We will briefly remind here. From 110 mg of Na2WO4 in 3 mL DI water, and 3 mL of 7% HNO3 aqueous solution, a H2WO4 deposition was formed instantaneously. Then, 16 mL of oleylamine was added to this H2WO4 deposition. To get clear solutions, the mixture was sonicated at room temperature for 20 min, transferred into a 45 mL autoclave, and purged with argon for 15 min. Afterward, the autoclave was put into an electronic oven at 220 °C during 12 h. The product was then deposited by adding 40 mL of ethanol and washed twice with absolute ethanol. Then, 10mg/mL of the resulting WO3−x NWs were dispersed in toluene. Finally, this solution with the nanomaterials was deposited by drop-casting 10 µL of the solutions (about 1 mg/mL) onto the electrodes. For this study, UV/O3 treatment during 15 min at 80 °C have been done before and after WO3−x NWs deposition to remove hydrocarbon contamination on the surface which became more hydrophilic with higher surface tension [7]. To be able to test the sensors up to 130 °C a heating was performed at 150 °C during 30 min on some samples.
Gas sensing investigates was performed at various temperatures under obscurity. A 1 V DC voltage was applied to the samples while the electrical resistance was monitored using a Keithley Model 2450 Source Meter (Tektronix, USA, Source Measure Unit). As the main purpose was first to check the WO3−x NWs gas-sensing properties using NH3 as target gas at low temperature, humidity was avoided using dry air as carrier gas. Because WO3 is n-type semiconductor and NH3 a reduction gas, the sensor response is defined as the following equation:
where Rdry air is the resistance under dry air and RNH3 is the resistance under NH3 exposure.
R = Rdry air /RNH3
The measurements have been performed with dry air as both the reference and the carrier gas, maintaining a constant total flow of 500 Standard Cubic Centimeters per Minute (SCCM) via mass flow controllers. The exposition time was fixed at 30 s.
3. Results and Discussion
The WO3−x NWs drop casted from a diluted solution on a mesh-coated carbon film presented a homogeneous length (about 100 nm) and shape dispersions (Figure 1a) observed by High-Resolution Transmission Electron Microscope (HR-TEM) JEOL-JEM 3010 (USA). Figure 1b presents the WO3−x NWs homogeneous layer formed on Si-SiO2 substrate. No response was measurable at 25 °C and 50 °C due to the high material resistivity and the high signal/noise ratio. Sensor responses were observed from 75 °C up to 130 °C since post-heating process was carry on at 150 °C.
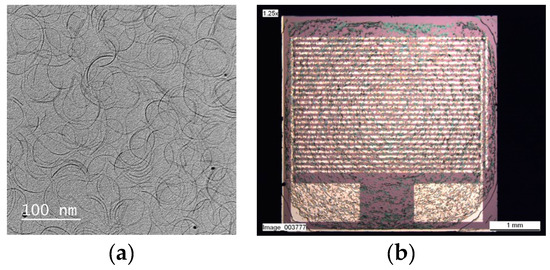
Figure 1.
(a) TEM image of WO3−x NWs and (b) Si-SiO2 substrate picture with WO3−x NWs dropped on it.
Figure 2 shows the representative dynamic gas response of WO3−x NWS to NH3 exposure with the concentrations of 2, 5, 10, 20 and 50 ppm at a working temperature equals to 130 °C. The resistance decrease induced by NH3 exposures on Figure 2 is in agreement with the behavior of a reduction gas on an n-type semiconductor. The mean idea is that the conductivity is controlled by oxygen vacancies which are generated and destroyed at the gaseous interface by reaction with oxygen.
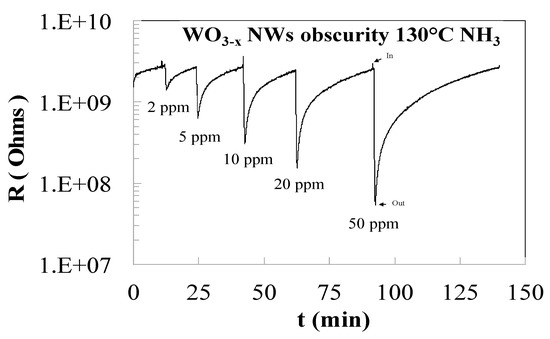
Figure 2.
Resistance variation of WO3−x NWs with NH3 exposure with the concentrations of 2, 5, 10, 20 and 50 ppm at a working temperature equals to 130 °C.
Before NH3 exposure, oxygen (O2) in the air will adsorb and react with WO3 on its interface. The charge carrier concentration and therefore the conductivity change in response to the oxygen vacancy concentration variations at the interface. In this process, O2 will capture electrons from WO3 and transform into negatively-charged species (Oσ− = 1/2, 1 or 2). During NH3 exposure, it will react with Oσ− to produce nitrogen or nitrogen oxides and electrons will return to WO3 [8]. The reactions at the interface develop a charge at the interface, which is balanced by a space charge of electrons within the oxide nanowire, near the interface. The surface-to-volume ratio of the nanowires will also increase the conductivity change and sensor sensitivity. The sensor response as a function of the NH3 concentration was plotted in Figure 3.
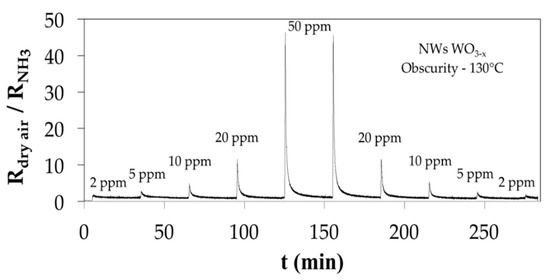
Figure 3.
Sensor response curve of WO3−x NWS based sensing device post-annealed at 150 °C and working at 130 °C in obscurity.
The response shows a nearly linear increase with the NH3 concentration rise. As illustrated in Figure 3, it was found a fast response less than 20 s and a time to return to the baseline around 5 min for an ammonia concentration of 2 ppm at 130 °C. This return time at this low temperature is interesting for using it several times in a short period. According to the sensor response curve presented in Figure 3, the WO3−x nanowires-based sensing device exhibits good reversible response at 130 °C without need of light excitation. The repeatability tests, by increasing and decreasing NH3 concentration exposures show a small deviation, an important response, a good reversibility, fast response and recovery without sensor saturation from 2 ppm to 50 ppm NH3 gas.
4. Conclusions
This study presents the efficiency of tungsten trioxide nanowires WO3−x deposited on rigid substrate by drop coating from colloidal solution to detect ammonia below the Recommended Exposure Limit Values (25 ppm) as low as 2 ppm at 130 °C without need of light excitation. This working temperature is compatible with thermal strain of flexible substrate. It is an attractive way to fabricate ammonia sensors on flexible substrate with interesting properties in terms of sensitivity and response times for applications in environmental monitoring devices. Further studies will be carry on selectivity versus other environmental gases and light illumination impact.
References
- Timmer, B.; Olthuis, W.; Van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Fine, G. F.; Cavanagh, L.M.; Afonja, A.; Russell, B. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Miura, N.; Yamazoe, N. Study of WO3-based sensing material for NH3 and NO detection. Sens. Actuators B 2000, 66, 74–76. [Google Scholar] [CrossRef]
- Wei, W.; Li, W.; Wang, L. High-selective sensitive NH3 gas senor: A density functional theory study. Sens. Actuators B 2018, 502–507. [Google Scholar] [CrossRef]
- Liu, J.; Margeat, O.; Dachraoui, W.; Liu, X.; Fahlman, M.; Ackermann, J. Gram-Scale,Synthesis of Ultrathin Tungsten Oxide Nanowires and their Aspect Ratio-Dependent Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 6029–6037. [Google Scholar] [CrossRef]
- Luo, S.; Wong, C.P. Effect of UV/ozone treatment on surface tension and adhesion in electronic packaging. IEEE Trans. Compon. Packag. Technol. 2001, 24, 43–49. [Google Scholar] [CrossRef]
- Jiménez, I.; Centeno, M.A.; Scotti, R.; Morazzoni, F.; Arbiol, J. NH3 interaction with chromium-doped WO3 nanocrystalline powders for gas sensing applications. J. Mater. Chem. 2004, 14, 2412–2420. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).