A Reliable and Sensitive Method Using Cyclic Voltammetry for the Detection of Airborne Fungi †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
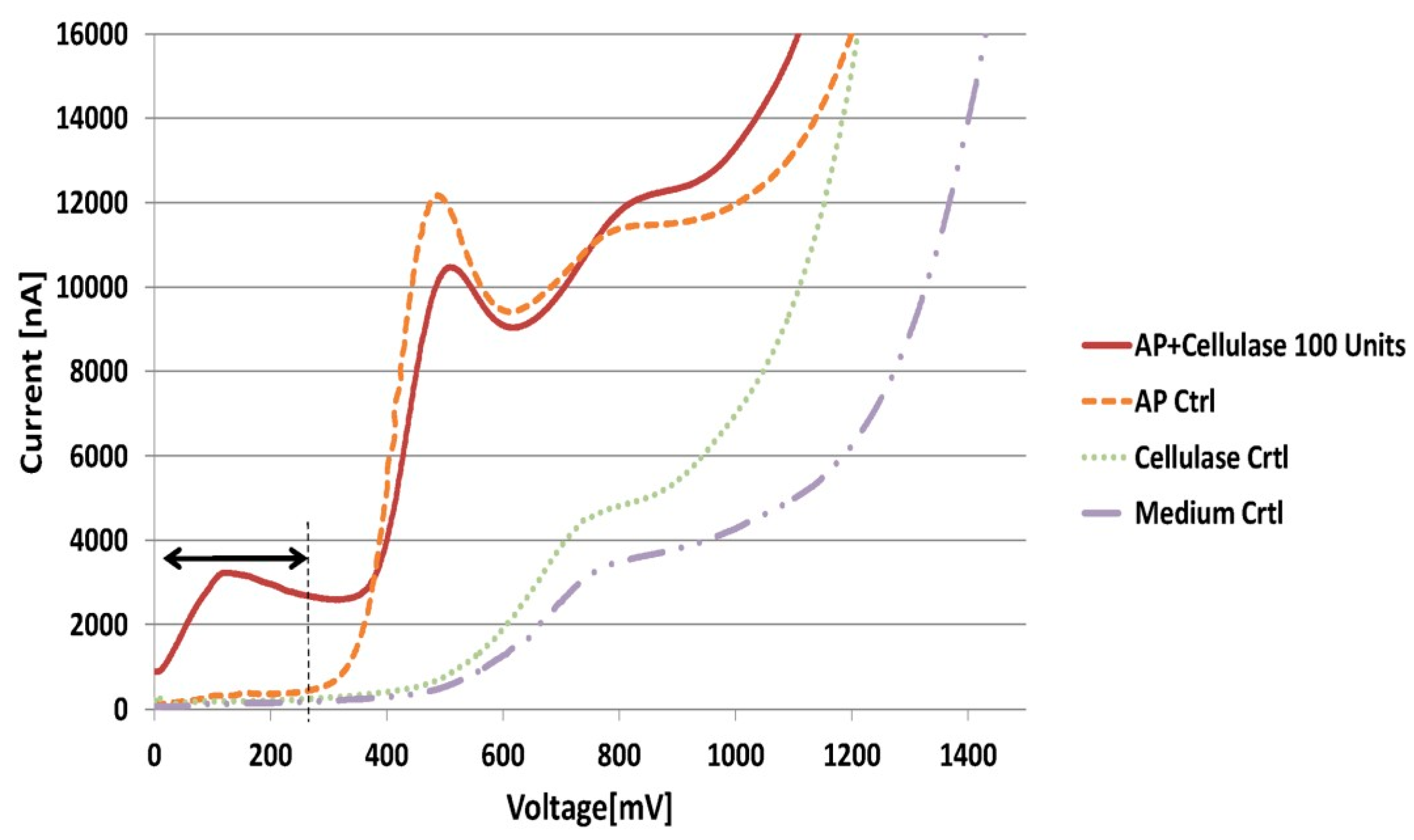
3.1. Proof of Principle
3.2. Temperature Optimization
3.3. Specificity and Cross Reactivity
Acknowledgments
Conflicts of Interest
References
- Commission of the European Communities. Biological Particles in Indoor Environments. In European Collaborative Action—Indoor Air Quality & its Impact on Man; Report No. 12; Commission of the European Communities: Brussels, Belgium, 1993. [Google Scholar]
- Cruys-Bagger, N.; Tatsumi, H.; Borch, K.; Westh, P. A graphene screen-printed carbon electrode for real-time measurements of unoccupied active sites in a cellulase. Anal. Biochem. 2014, 447, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Mikus, M.; Schuster, A.; Schmoll, M.; Seiboth, B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol. Biofuels 2009, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Kellner, K.H.; Posnicek, T.; Ettenauer, J.; Zuser, K.; Brandl, M. A new, low-cost potentiostat for environmental measurements with an easy-to-use PC interface. Procedia Eng. 2015, 120, 956–960. [Google Scholar] [CrossRef]
- Sohail, M.; Siddiqi, R.; Ahmad, A.; Khan, S.A. Cellulase production from Aspergillus niger MS82: Effect of temperature and pH. New Biotechnol. 2009, 25, 437–441. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettenauer, J.; Zuser, K.; Pfeiffer, S.; Cserna, D.; Brandl, M. A Reliable and Sensitive Method Using Cyclic Voltammetry for the Detection of Airborne Fungi . Proceedings 2018, 2, 959. https://doi.org/10.3390/proceedings2130959
Ettenauer J, Zuser K, Pfeiffer S, Cserna D, Brandl M. A Reliable and Sensitive Method Using Cyclic Voltammetry for the Detection of Airborne Fungi . Proceedings. 2018; 2(13):959. https://doi.org/10.3390/proceedings2130959
Chicago/Turabian StyleEttenauer, Jörg, Karen Zuser, Sandra Pfeiffer, Dominique Cserna, and Martin Brandl. 2018. "A Reliable and Sensitive Method Using Cyclic Voltammetry for the Detection of Airborne Fungi " Proceedings 2, no. 13: 959. https://doi.org/10.3390/proceedings2130959
APA StyleEttenauer, J., Zuser, K., Pfeiffer, S., Cserna, D., & Brandl, M. (2018). A Reliable and Sensitive Method Using Cyclic Voltammetry for the Detection of Airborne Fungi . Proceedings, 2(13), 959. https://doi.org/10.3390/proceedings2130959




