The Effect of Pt Decoration on the Gas Sensing Properties of Copper Oxide Nanorods †
Abstract
:1. Introduction
2. Experimental
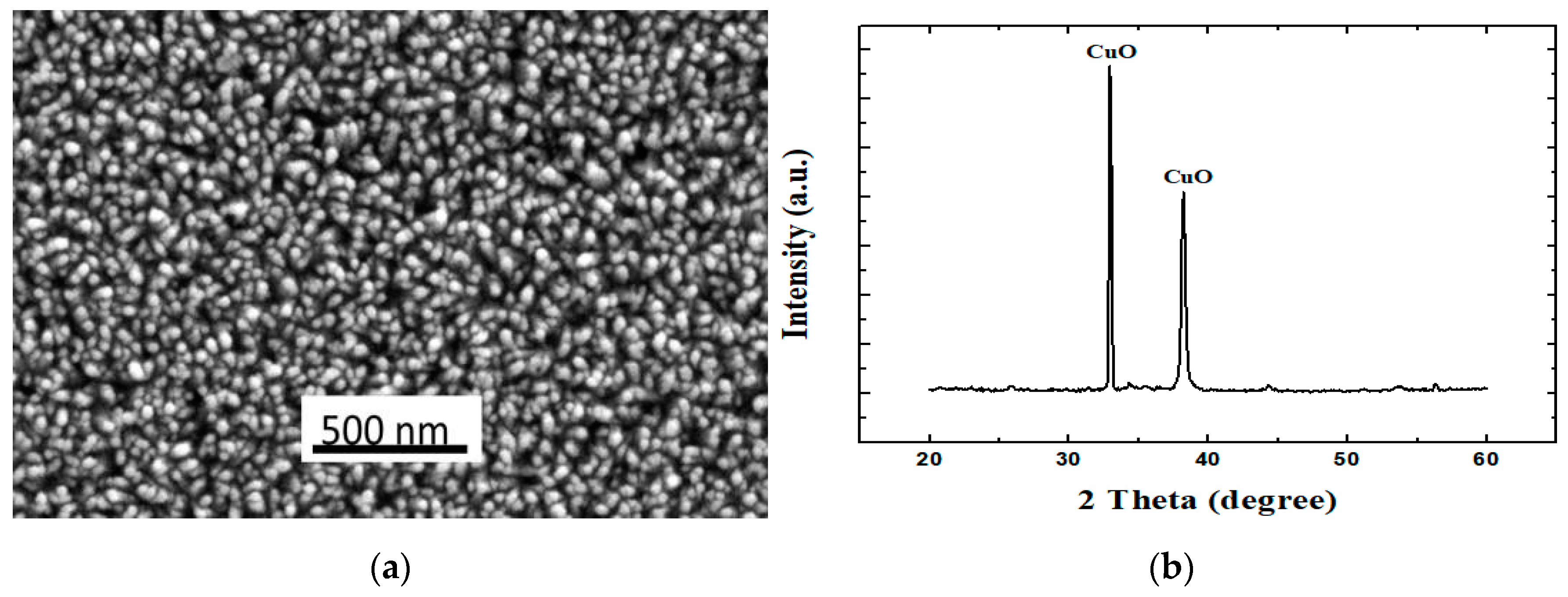
2.1. Synthesis and Characterization of Cuo Nanorods
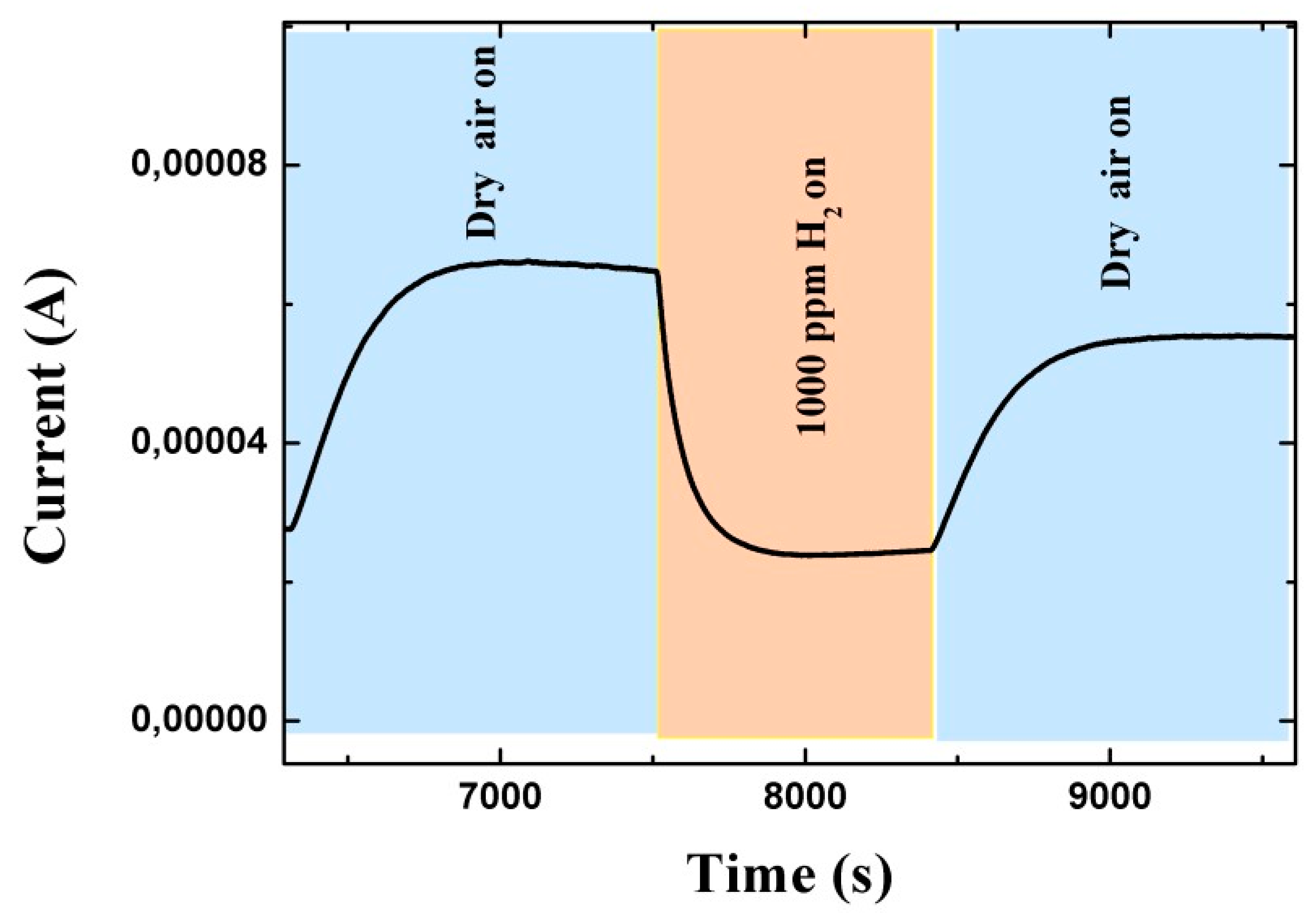
2.2. Sensor Measurements
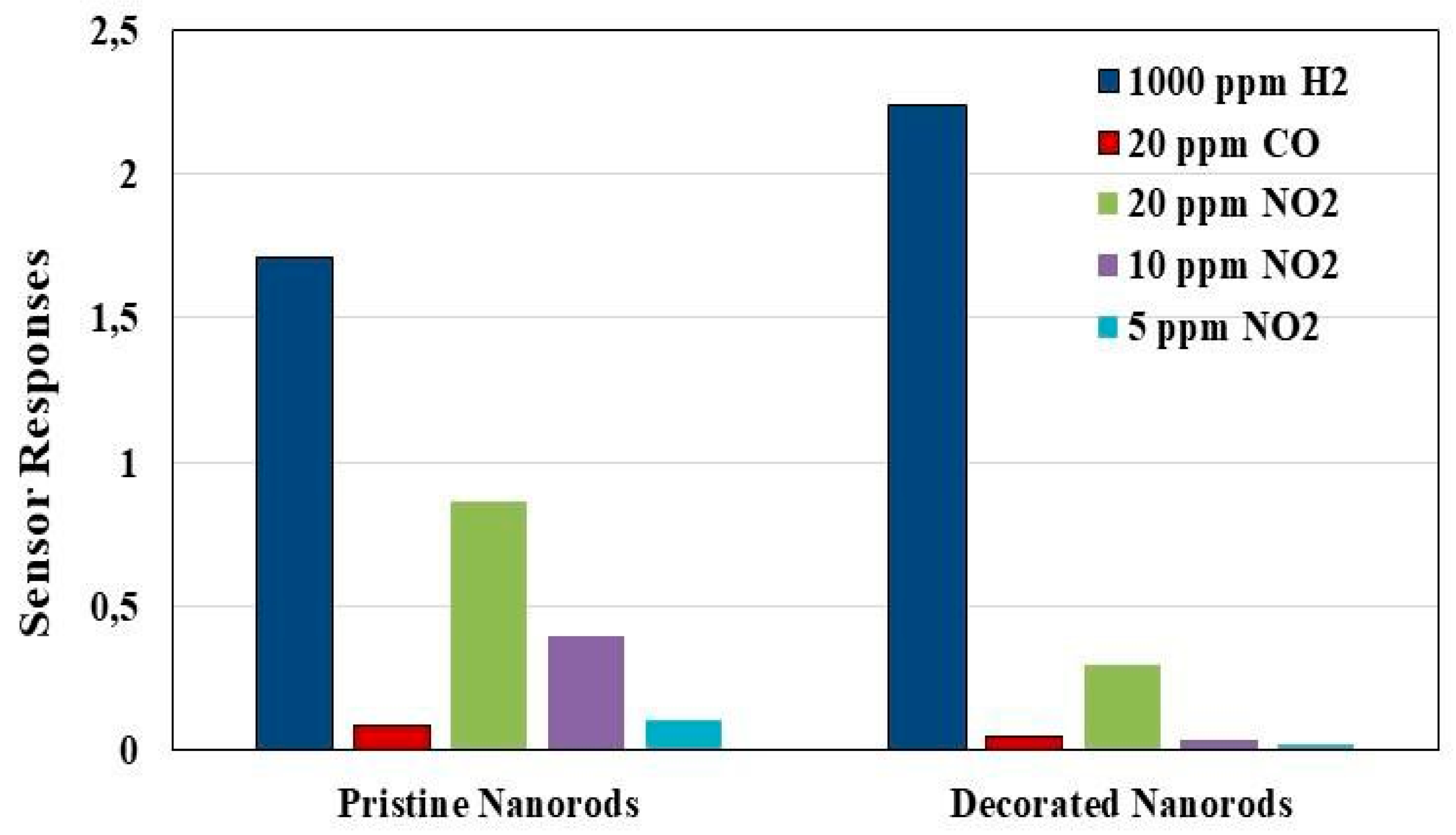
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Şişman, O.; Kılınç, N.; Öztürk, Z.Z. Structural, electrical and H2 sensing properties of copper oxide nanowires on glass substrate by anodization. Sens. Actuators B Chem. 2016, 236, 1118–1125. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.R.; Sberveglieri, G. Preparation of copper oxide nanowire-based conductometric chemical sensors. Sens. Actuators B Chem. 2013, 182, 7–15. [Google Scholar] [CrossRef]
- Liu, L.; Hong, K.; Hu, T.; Xu, M. Synthesis of aligned copper oxide nanorod arrays by a seed mediated hydrothermal method. J. Alloys Compd. 2012, 511, 195–197. [Google Scholar] [CrossRef]
- Şişman, O.; Kılınç, N.; Öztürk, Z.Z. H2 Sensing Properties of Cu2O Nanowires on Glass Substrate. Procedia Eng. 2015, 120, 1170–1174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıca, N.; Alev, O.; Öztürk, Z.Z. The Effect of Pt Decoration on the Gas Sensing Properties of Copper Oxide Nanorods. Proceedings 2018, 2, 956. https://doi.org/10.3390/proceedings2130956
Sarıca N, Alev O, Öztürk ZZ. The Effect of Pt Decoration on the Gas Sensing Properties of Copper Oxide Nanorods. Proceedings. 2018; 2(13):956. https://doi.org/10.3390/proceedings2130956
Chicago/Turabian StyleSarıca, Neslihan, Onur Alev, and Zafer Ziya Öztürk. 2018. "The Effect of Pt Decoration on the Gas Sensing Properties of Copper Oxide Nanorods" Proceedings 2, no. 13: 956. https://doi.org/10.3390/proceedings2130956
APA StyleSarıca, N., Alev, O., & Öztürk, Z. Z. (2018). The Effect of Pt Decoration on the Gas Sensing Properties of Copper Oxide Nanorods. Proceedings, 2(13), 956. https://doi.org/10.3390/proceedings2130956




