Abstract
In this paper we report on the development tungsten oxide based chemiresistive sensors for the monitoring of oxygen at low temperatures (T ≤ 400 °C) in dry and humid air. The sensors were deposited onto alumina substrate by a combination of spin coating and a photolithographic process to define the sensing area. Our results show that the sensors comply with a linear relationship over a 0 to 20% concentration range, with a high response towards oxygen. The highest response was observed at 350 °C (ΔR/Ra = 7.8) in humid and in dry air (ΔR/Ra = 18). This result is a significant improvement over our previous experiments and we believe to take the concept of a metal-oxide based oxygen sensor a step closer.
1. Introduction
Oxygen monitoring has been widely used by the automotive industry, for medical applications, for food and beverage packaging and in fire prevention systems. Currently, these markets are dominated by electrochemical based gas sensors that rely on a Pb-anode. This type of sensor has a limited life-span as the sensor will fail when all the lead has been oxidised. Moreover, the “Restriction of Hazardous Substances Directive” (RoHS), which bans the use of lead in almost all electronic products, is likely to be applied to gas sensors by 2024 at the latest. This leads to the requirement of lead-free oxygen sensor.
Here we propose the use of an inorganic metal-oxide (MOX) material for the development of a compact and ultra-low cost oxygen sensor, as an alternative to Pb-based devices. The advantages of MOX sensors include high sensitivity, low manufacturing cost, ability to operate in harsh environments and simple resistive measurement. In this work, tungsten oxide powders were prepared by a spin coating method onto ceramic tiles, followed by a photolithographic definition step, to make thin-film sensors.
2. Experimental
2.1. Sensor Fabrication
The sensors were produced as thin films by spin coating method added with photolithographic process to define the sensing area. Ink was prepared in a yellow light room from a 3:1 mixture of negative photoresist Dirasol 22 (Fujifilm Sericol Ltd., UK) and tungsten oxide powder (Treibacher Industrie Ag, Austria). A small quantity of deionized (DI) water was added to the mixture to reduce the viscosity. The mixture was placed in a ceramic pot and milled for 35 min using Laboratory Fast Mill Mod. Speedy (Nannetti srl, Italy) to create a smooth and consistent ink.
Ink was deposited on alumina substrates on top of previously deposited gold interdigitated electrodes using Spin Coater G3P-8 (Specialty Coating Systems, USA) at 3000 rpm for 30 s. The substrates were dried at 55 °C for 5 min on a hot plate followed by 5 min ultraviolet exposure through a mask. DI water was used as the developer to remove the unexposed part of the substrate, leaving only the exposed part intact. Subsequently, the substrates were placed on hot plate until they were completely dried. After this, the whole deposition process was repeated to create a 2-layer film before sintered at 400 °C for 2 h and ramped up to 900 °C for 1.5 h. The substrates were then cooled down to room temperature.
2.2. Gas Sensing Tests
Experiments were performed using a gas mixture instrument previously built in the School of Engineering University of Warwick, UK. The instrument uses 2 MFCs controlled by a software program written in LabVIEW (National Instrument 2016, USA) to produce different oxygen concentrations by diluting zero air (20% O2) with 99.999% N2 (Lehman Instrument, France). Humid air was obtained by passing the mixture gas through a water bubbler, which provides humidity at about 85%, monitored using a data logger (Lascar Electronics, UK), before entering an air-tight chamber where the gas sensors are housed. For experiments in dry air, the water bubbler was omitted. The sensor resistance change upon exposure to different oxygen concentration was monitored and logged using a Sensor Management System AS-330 (Atmospheric Sensor Ltd., UK). With this instrument, temperature changes of the sensors can also be controlled. Here, we used temperature ranges between 150 °C and 400 °C. The fabricated sensors were exposed to different oxygen concentration for 30 min and subsequently purged with N2 for about 30 min to allow sensor recover to its baseline. This process is done before any new measurements were taken, especially at different temperature. The sensor response was defined as (Rg − Ra)/Ra or ΔR/Ra where Ra represents the sensor baseline resistance in reference gas N2 and Rg is the resistance of the sensor upon contact with targeted oxygen concentration in the reference gas.
3. Results and Discussion
Gas-sensing tests were carried out to detect 20% oxygen at various temperatures from 150 °C to 400 °C to examine the effect of temperature on sensor performance. Figure 1 shows the sensor responses to 20% O2 as a function of operating temperature. At temperature less than 200 °C, sensors exhibited almost no response to changes in oxygen level. As the operating temperature increased, sensor responses also increased with the maximum achieved at 350 °C (ΔR/Ra = 18 in dry and ΔR/Ra = 7.8 in wet air). Temperature higher than 350 °C lead to a decrease in sensor response. This is likely due to higher temperatures causing oxygen desorption rather than the adsorption [1,2,3]. Here we observe throughout the temperature ranges, the films exhibited higher response in dry than in wet air.
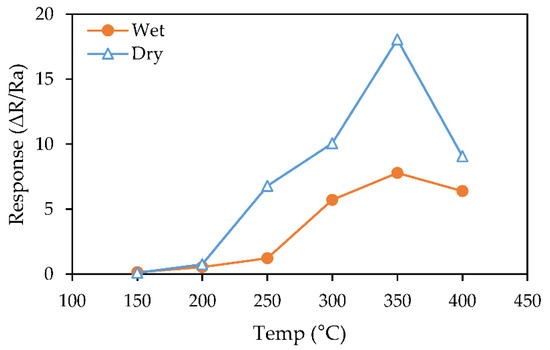
Figure 1.
Sensor responses to 20% O2 as a function of the operating temperature in dry vs. wet air (85% RH).
Figure 2 shows the sensor dynamic response to various oxygen concentration at 350 °C. At this temperature, the baseline temperature was found to be around 30–40 kΩ. The maximum response (20% O2) was noted at 0.9 MΩ in dry air compared to 0.3 MΩ in wet air. As observed in Figure 2, the resistance of thin films in N2 ambience (baseline resistance) was unaffected by humidity. On the other hand, the resistance decreased to a third in the presence of humidity and so resulted in a lower response. Previous studies on the effect of humidity on sensor performance reported similar findings where reduced response was observed when humidity was introduced in the environment [4,5,6]. This is due to the targeted gas competing with water molecules to occupy the active sites on the surface of the sensing material [7,8,9]. Nonetheless, our sensors demonstrated a good response in both dry and humid environment.
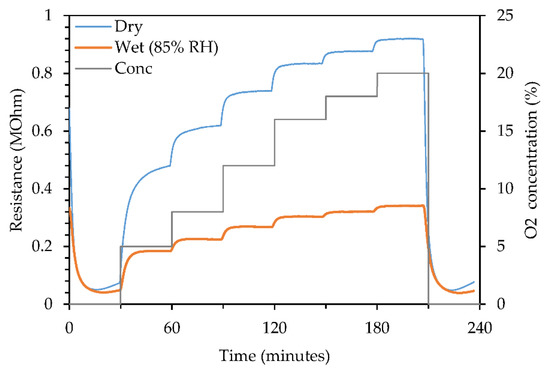
Figure 2.
Sensor dynamic responses to various O2 concentration at 350 °C.
The gas response relationship is illustrated in Figure 3 shows that the response changes linearly with the changes in oxygen concentration, which can ease its use in the future applications. The results also showed a significant improvement over our previous work on WO3 based sensor for oxygen detection deposited via AACVD [10].
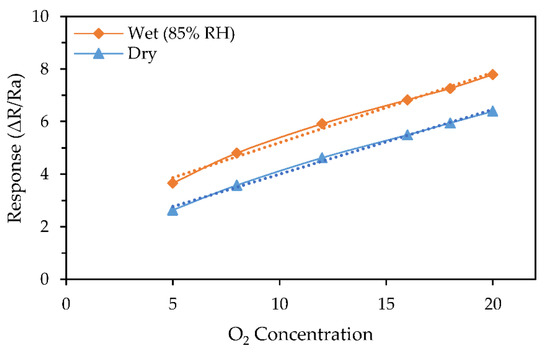
Figure 3.
Sensor responses toward various oxygen concentration in dry and wet air (85% RH).
4. Conclusions
Tungsten oxide thin films have been successfully deposited onto alumina substrate by spin coating method with photolithographic step to define the sensing area. The sensors were tested in dry and wet air with operating temperature ranging from 150–400 °C. The results showed the sensors had a good response to changes oxygen concentration, with the maximum response observed at 350 °C. Here, we demonstrated the humidity affected sensor performance but the sensor response in wet air was still useable. The sensor resistance changed linearly with oxygen concentration, which could simplify its use for future applications.
Author Contributions
W.P. fabricated the sensor, and performed the experiment. S.L. contributed the instruments used to fabricate the sensor, J.C. oversaw the process from sensor fabrication to data analysis.
Acknowledgments
The study is supported by Lembaga Pengelola Dana Pendidikan, Ministry of Finance, Republic of Indonesia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gurlo, A. Interplay between O2 and SnO2: oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, K.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. Low-temperature hydrothermal synthesis of WO3 nanorods and their sensing properties for NO2. J. Mater. Chem. 2012, 22, 12643–12650. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bhatnagar, M.C.; Sharma, G.L. Mechanism of highly sensitive and fast response Cr doped TiO2 oxygen gas sensor. Sens. Actuators B Chem. 1997, 45, 209–215. [Google Scholar] [CrossRef]
- Gong, J.; Chen, Q.; Lian, M.-R.; Liu, N.-C.; Stevenson, R.G.; Adami, F. Micromachined nanocrystalline silver doped SnO2 H2S sensor. Sens. Actuators B Chem. 2006, 114, 32–39. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Zheng, X.; Fan, H.; Liu, L.; Wang, R.; Zeng, Y. Electrical response of Sm2O3-doped SnO2 to C2H2 and effect of humidity interference. Sens. Actuators B Chem. 2008, 134, 36–42. [Google Scholar] [CrossRef]
- Egashira, M.; Nakashima, M.; Kawasumi, S.; Selyama, T. Temperature programmed desorption study of water adsorbed on metal oxides. 2. Tin oxide surfaces. J. Phys. Chem. 1981, 85, 4125–4130. [Google Scholar] [CrossRef]
- Shankar, P.; Bosco, J.; Rayappan, B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; Di Maggio, F.; Vallejos, S.; Vilic, T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-Assisted CVD-Grown PdO Nanoparticle-Decorated Tungsten Oxide Nanoneedles Extremely Sensitive and Selective to Hydrogen. ACS Appl. Mater. Interfaces 2016, 8, 10413–10421. [Google Scholar] [CrossRef] [PubMed]
- Annanouch, F.E.; Haddi, Z.; Vallejos, S.; Umek, P.; Guttmann, P.; Bittencourt, C.; Llobet, E. Aerosol-Assisted CVD-Grown WO3 Nanoneedles Decorated with Copper Oxide Nanoparticles for the Selective and Humidity-Resilient Detection of H2S. ACS Appl. Mater. Interfaces 2015, 7, 6842–6851. [Google Scholar] [CrossRef] [PubMed]
- Sari, W.P.; Blackman, C.; Zhu, Y.; Covington, J. Deposition of tungsten oxide and silver decorated tungsten oxide for use in oxygen gas sensing. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).