Preparation of Calcium Carbonate Nanoparticles: Adsorption and Desorption Behavior of Organic Matter †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CaCO3 Nanoparticles
2.2. Preparation Synthetic Organic Matter Solution and Adsorption Studies
3. Results and Discussion
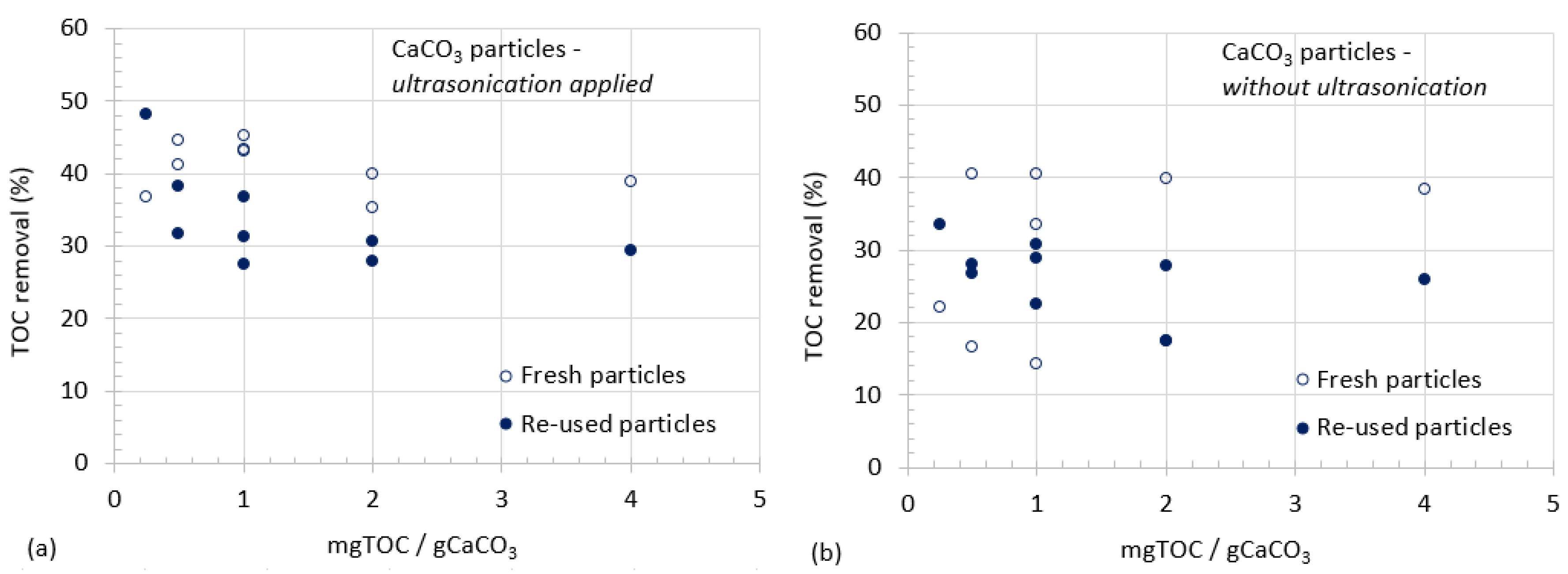
3.1. CaCO3 Particle Characterization
3.2. Organic Matter Removal
4. Conclusions
- Laboratory made calcium carbonate (CaCO3) particles were considered as a medium for the removal of organic matter from waters.
- Two different particles were prepared and their physical properties were determined. These are: the particles used in organic matter removal as they were prepared, and the particles used in organic matter removal after ultrasonication following their preparation.
- Ultrasonicated particles demonstrated better performances than particles used without any dispersal or other effects of ultrasonication especially for the removal of relatively lower concentrations of organics.
- Experiments were conducted for the investigation of adsorption capacity of particles during repeated use.
- Results obtained in this study would be a promising solution strategy for the problem of management of concentrated flows collected in the treatment concentrates of membrane filtration plants.
Author Contributions
Funding
Conflicts of Interest
References
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G.; Borchardt, J.H. Physical and Chemical Quality of Water. In MWH’s Water Treatment. Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Edzwald, J.K.; Tobiason, J.E. Chemical Principles, Source Water Composition, and Watershed Protection. In Water Quality & Treatment. A Handbook on Drinking Water, 6th ed.; Edzwald, J.K., Ed.; McGraw Hill: New York, NY, USA, 2011. [Google Scholar]
- Li, S.; He, M.; Li, Z.; Li, D.; Pan, Z. Removal of humic acid from aqueous solution by magnetic multi-walled carbon nanotubes decorated with calcium. J. Mol. Liq. 2017, 230, 520–528. [Google Scholar] [CrossRef]
- Bob, M.; Walker, H.W. Enhanced adsorption of natural organic matter on calcium carbonate particles through surface charge modification. Colloids Surf. Physicochem. Eng. Asp. 2001, 191, 17–25. [Google Scholar] [CrossRef]
- Sudoh, R.; Islam, M.S.; Sazawa, K.; Okazaki, T.; Hata, N.; Taguchi, S.; Kuramitz, H. Removal of dissolved humic acid from water by coagulation method using polyaluminum chloride (PAC) with calcium carbonate as neutralizer and coagulant aid. J. Environ. Chem. Eng. 2015, 3, 770–774. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Saini, D.R.; Pandit, A.B. Continuous precipitation of calcium carbonate using sonochemical reactor. Ultrason. Sonochem. 2015, 24, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Standard Methods. 2540 Solids, 2340 Hardness. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]






| Organic Matter Loading (mg TOC/g CaCO3) | ||||
|---|---|---|---|---|
| TOC (mg/L) | 25 | 0.25 | 0.5 | 1 |
| 50 | 0.5 | 1 | 2 | |
| 100 | 1 | 2 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolfagharian, P.; Arslan, G.; Yiğit-Hunce, S.; Soyer, E. Preparation of Calcium Carbonate Nanoparticles: Adsorption and Desorption Behavior of Organic Matter. Proceedings 2018, 2, 655. https://doi.org/10.3390/proceedings2110655
Zolfagharian P, Arslan G, Yiğit-Hunce S, Soyer E. Preparation of Calcium Carbonate Nanoparticles: Adsorption and Desorption Behavior of Organic Matter. Proceedings. 2018; 2(11):655. https://doi.org/10.3390/proceedings2110655
Chicago/Turabian StyleZolfagharian, Payam, Gülay Arslan, Selda Yiğit-Hunce, and Elif Soyer. 2018. "Preparation of Calcium Carbonate Nanoparticles: Adsorption and Desorption Behavior of Organic Matter" Proceedings 2, no. 11: 655. https://doi.org/10.3390/proceedings2110655
APA StyleZolfagharian, P., Arslan, G., Yiğit-Hunce, S., & Soyer, E. (2018). Preparation of Calcium Carbonate Nanoparticles: Adsorption and Desorption Behavior of Organic Matter. Proceedings, 2(11), 655. https://doi.org/10.3390/proceedings2110655




