Removal of Nonylphenol and Octylphenol from Aqueous Solutions by a Novel Nano-Composite (ZVI/Fullerene) †
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Solutions
2.2. GC-MS Analysis
2.3. Synthesis of Iron Nanoparticles (nZVI)
2.4. Preparation of Fullerene Nanoparticles (aq-nC60) and Preparation of nZVI/aq-nC60 Nanocomposite
2.5. Experimental Setup
3. Results and Discussion
3.1. Characterization of Nanoparticles
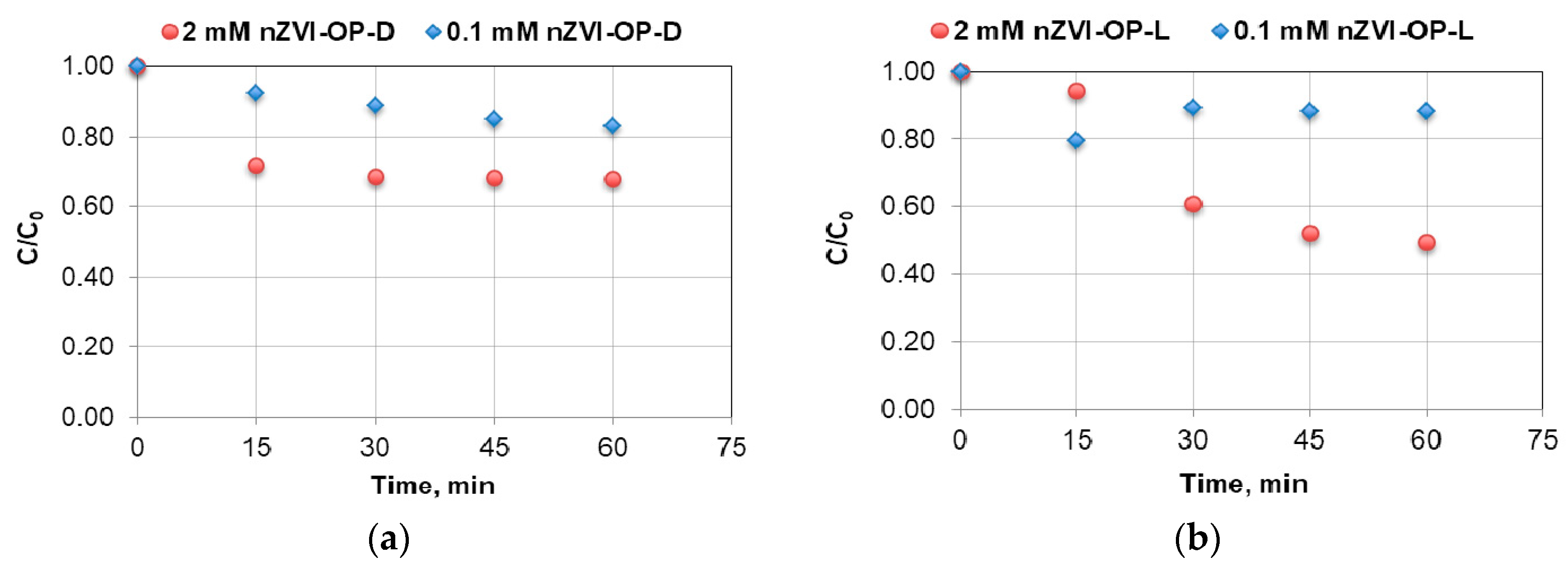
3.2. Effect of Iron Nanoparticle (nZVI) Concentration on the Removal of Nonylphenol and Octylphenol
3.3. Effect of Light on Removal of Nonylphenol and Octylphenol
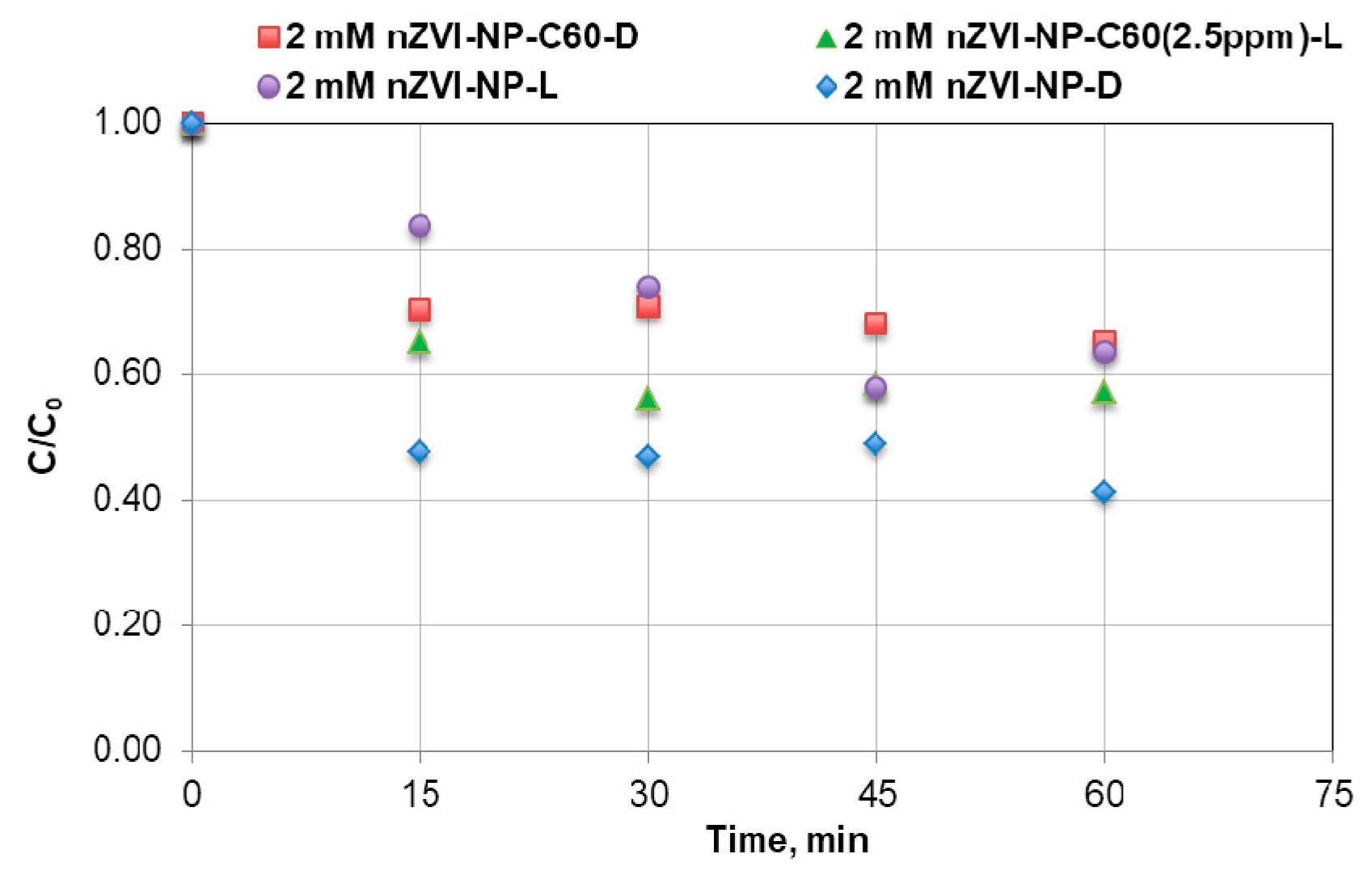
3.4. Effect of aq/nC60-nZVI Composite on Nonylphenol and Octylphenol Removal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Servos, M.R. Review of the aquatic toxicity, estrogenic responses and bioaccumulation of alkylphenols and alkylphenol polyethoxylates. Water Qual. Res. J. Can. 1999, 34, 123–177. [Google Scholar] [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.; Mihaich, E.; Carbone, J.; Woodburn, K.; Klecka, G. A weight of evidence analysis of the chronic ecotoxicity of nonylphenol ethoxylates, nonylphenol ether carboxylates, and nonylphenol. Hum. Ecol. Risk Assess. 2004, 10, 999–1017. [Google Scholar] [CrossRef]
- Can, Z.S.; Fırlak, M.; Kerç, A.; Evcimen, S. Evaluation of different wastewater treatment techniques in three wwtps in istanbul for the removal of selected edcs in liquid phase. Environ. Monit. Assess. 2014, 186, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Sun, H.; Xu, J.; Dai, S. Distribution and dissipation pathways of nonylphenol polyethoxylates in the yellow river: Site investigation and lab-scale studies. Environ. Int. 2006, 32, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Heidler, J.; Halden, R.U. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ. Sci. Technol. 2008, 42, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Hu, J.; An, W.; Yang, M. Detection and occurrence of chlorinated byproducts of bisphenol a, nonylphenol, and estrogens in drinking water of china: Comparison to the parent compounds. Environ. Sci. Technol. 2013, 47, 10841–10850. [Google Scholar] [CrossRef] [PubMed]
- Erdim, E.; Badireddy, A.R.; Wiesner, M.R. Characterizing reactive oxygen generation and bacterial inactivation by a zerovalent iron-fullerene nano-composite device at neutral ph under UV-a illumination. J. Hazard. Mater. 2015, 283, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Chen, Z.; Yang, H.; Yang, L.; Luo, L.; Chen, C. The transformation of corrosion products on weathering steel by visible-light illumination under simulated marine atmospheric condition. Int. J. Electrochem. Sci. 2016, 11, 10498–10510. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haciosmanoglu, G.G.; Yücesoy-Ozkan, Z.; Can, Z.S.; Genc, S.; Soyer, E.; Pehlivanoglu-Mantas, E.; Erdim, E. Removal of Nonylphenol and Octylphenol from Aqueous Solutions by a Novel Nano-Composite (ZVI/Fullerene). Proceedings 2018, 2, 654. https://doi.org/10.3390/proceedings2110654
Haciosmanoglu GG, Yücesoy-Ozkan Z, Can ZS, Genc S, Soyer E, Pehlivanoglu-Mantas E, Erdim E. Removal of Nonylphenol and Octylphenol from Aqueous Solutions by a Novel Nano-Composite (ZVI/Fullerene). Proceedings. 2018; 2(11):654. https://doi.org/10.3390/proceedings2110654
Chicago/Turabian StyleHaciosmanoglu, Gül Gülenay, Zeynep Yücesoy-Ozkan, Zehra Semra Can, Seval Genc, Elif Soyer, Elif Pehlivanoglu-Mantas, and Esra Erdim. 2018. "Removal of Nonylphenol and Octylphenol from Aqueous Solutions by a Novel Nano-Composite (ZVI/Fullerene)" Proceedings 2, no. 11: 654. https://doi.org/10.3390/proceedings2110654
APA StyleHaciosmanoglu, G. G., Yücesoy-Ozkan, Z., Can, Z. S., Genc, S., Soyer, E., Pehlivanoglu-Mantas, E., & Erdim, E. (2018). Removal of Nonylphenol and Octylphenol from Aqueous Solutions by a Novel Nano-Composite (ZVI/Fullerene). Proceedings, 2(11), 654. https://doi.org/10.3390/proceedings2110654



