Abstract
Sulphide was adopted as odorous compound in a simulation of AS Diffusion, an interesting process to treat odors at wastewater treatment plants by diffusing odorous air into aerobic basins. Its behaviour were experimentally evaluated along with its effects on the biomass and the biological processes supposed by some author in an AS diffusion test. Two bench scale sequencing batch reactors (SBRs) were fed in parallel on real primary sewage and monitored after adding increasing concentrations of sulphide to one of them. In this reactor, an average sulphide removal of 94% was measured. Microbial biochemical activity and composition did not show relevant variations after the addition of sulphide, and the good features of activated sludge flocs were maintained also in terms of sludge settleability.
1. Introduction
Many reduced sulphur compounds can be used as electron donors by a variety of bacteria having relevance in wastewater treatment. The most common sulphur compound used as energy source is hydrogen sulphide (H2S) that is oxidized by microorganisms to elemental sulphur, up to sulphate, mainly in presence of molecular oxygen [1]. The biological oxidation of sulphide is used in the process called “activated sludge diffusion” (AS diffusion) to treat odorous air at wastewater treatment plants, since odors associated with sewage and wastewater treatment are mainly generated by H2S [2,3,4,5,6]. In biological reactors equipped for AS diffusion [7], the gaseous Sulphur compounds diffuse into the liquid phase where microorganisms are able to oxidize them.
Among these oxidizing bacteria, there are some filamentous microorganisms that are of critical importance in wastewater treatment, because they are responsible for bulking of the activated sludge [8,9]. These were morphologically characterized as Beggiatoa spp., Thiothrix spp., type 021N and type 0914 [10,11]. Hydrogen sulphide, especially when present in relatively high concentrations, can selectively favour the growth of these microorganisms with consequent solid separation problems [11]. High concentrations of sulphide can also cause inhibition of ammonia nitrification, although the specific mechanisms of this inhibition are still unclear [2].
Aim of this work was to evaluate the impact of H2S on activated sludge biomass with special reference to microbial composition and biochemical activities.
2. Materials and Methods
Two bench-scale sequencing batch reactors (SBRs), with a working volume of 3.0 L, were inoculated with identical mixed liquor and fed in parallel on domestic clarified sewage. The two reactors were continuously operated for 243 days in cycles of 8 h according to the operating phases shown in Figure 1.
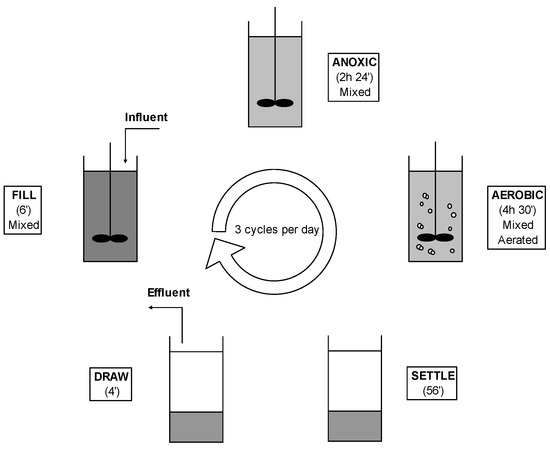
Figure 1.
Operating sequences of SBR system during one complete cycle (8 h).
Anoxic conditions were kept during Mixed fill and Mixed react steps to achieve denitrification. During the Draw step of each cycle, 1.8 L of treated effluent were discharged and replaced during the following Fill step with an equal volume of feed (stored at 4 °C), resulting in hydraulic retention time (HRT) of 13 h 20 min and a volumetric exchange ratio (VER) of 0.6. A sludge retention time (SRT) of 18 days was achieved with a manual removal of exceed sludge finalized to maintain a constant concentration of mixed liquor suspended solids (MLSS) of 3 g L−1.
The organic loading rate was variable depending on the characteristics of influent sewage with an average value of 0.2 kgCOD kgMLSS−1 d−1. Aerobic conditions were attained during Aerated react step with a residual dissolved oxygen (DO) concentration of 1–3 mg L−1 during the feast period [12]. Settling characteristics of the sludge were expressed as sludge volume index (SVI) (IRSA Bari-Italy, 1984) and temperature, dissolved oxygen (DO) and pH values were daily measured to monitor physical-chemical parameters of the process. One of the two biomasses was kept under selective pressure of hydrogen sulphide, in order to study the biochemical and microbiological effects of AS Diffusion. For this purpose, one of the two reactors (SBR1) was fed with an addition of a liquid solution of sodium sulphide (Na2S) during the first 4 h of the Aerated react step, with a volumetric loading rate of 17.03 mgS L−1 reactor d−1. This loading rate corresponds to a gas-phase concentration of 240 mgS (Nm3)–1, i.e., about twice the maximum value found in the literature on municipal wastewaters [5,13]. The two reactors were identical except this additional feeding. The headspace of each reactor (0.5 L) was connected to a trap containing a concentrated solution of zinc acetate, so that the sulphide escaping the reactor as a gas precipitated in the trap as zinc sulphide (ZnS).
2.1. Chemical Analysis
Chemical oxygen demand (COD), metabolic balances of nitrogen and sulphur were monitored by chemical characterization of the reactors’ influent and effluent.
COD, ammonia, nitrite and sulphate concentrations were determined according to Italian standard methods [14,15]. Nitrate, sulphide and total sulphur concentrations were determined according to standard methods [15] as well as mixed liquor suspended solids and volatile suspended solids (VSS).
2.2. Microscopic Analysis
The microbial composition of the mixed biomasses was investigated through microscopic observations with special focus on the characterization of filamentous organisms. Characterization of the filamentous microorganisms and description of sludge microscopic properties was performed according to Jenkins’ manual [51]. The method of subjective scoring of filament abundance, proposed by Jenkins [11], was applied to value the amount of filamentous bacteria. Activated sludge samples was periodically (once every Sludge Retention Time—SRT) collected from both reactors during the whole experimentation period up to a total number of 14 samples. Stains (Gram and Neisser stains) [11] were performed as well the test for sulphur oxidation (S Test) [11]. Microscopic observations were made with an Olympus BX 50 phase contrast microscope equipped with a digital camera. The introduction should briefly place the study in a broad context and define the purpose of the work and its significance. For papers that report original research, you should use the titles “Materials and Methods”, “Results”, “Discussion” and “Conclusions” (optional).
3. Results and Discussion
After a start-up period of 60 days, the two SBRs were operated in steady state under identical conditions until 147th day (Period 1) when the Na2S dosing system was turned on to feed the reactor SBR1 with a sulphide addition (Period 2) and the other one reactor (SBR2) operated as a control reactor (blank system).
In Table 1 are reported the main parameters, representative of biochemical activities in the system, during the two experimental periods (before and after the external addition of Na2S) and shows the performance of the two reactors in terms of COD and nitrogen removal efficiencies.

Table 1.
Average concentrations and removal efficiencies of the main process parameters.
The average COD concentration of the influent wastewater during the two periods was about 390 and 350 mgCOD L−1 respectively, and the removal efficiencies of the two parallel systems were about 90% in the first period. During the second period, when SBR1 was added with Na2S, the COD removal efficiency decreased for both reactors, and this was attributed to a lower quality of the influent wastewater. Therefore, COD and so the oxidative activity of heterotrophic population does not seem to be affected by sulphide addition.
No relevant differences were observed with respect to nitrification and denitrification, either between the two periods for reactor SBR1, or between the two reactors. Therefore, both autotrophic nitrifiers and heterotrophic denitrifiers would not seem to be affected by the sulphide externally fed to SBR1. This is in contrast with the findings of other researchers, who noted inhibition of nitrification possibly due to the presence of sulphide [2].
As regard to sulphide oxidation, the efficiency of its removal was about 93% in SBR1 and 77% in SBR2 (average values) and since no significant differences between the effluent sulphide concentrations were observed in the two reactors, a complete depletion of the external dose provided to SBR1 is suggested.
The amount of effluent gaseous sulphur accumulated in the traps was 0.07 mgS d−1 for reactor SBR1 and 0.08 mgS d−1 for reactor SBR2 (average values). Also in this case the two reactors behaved in similar ways, confirming that the external sulphur addition did not cause detectable differences in the liquid and gaseous effluents.
Figure 2 shows the mass balance of sulphur compounds and suggest no relevant differences between the two reactors. Most of the influent sulphide was oxidised to sulphate and left the plant in the effluent. The remaining sulphide was partly found in the effluent and a very limited amount was stripped. A certain amount of sulphur accumulated in the mixed liquor and was removed from the plant with the waste sludge, as pointed out by other authors [8].
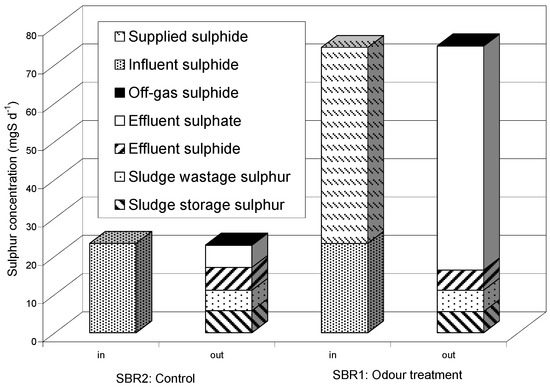
Figure 2.
Mass balance of main sulphur compounds during the second period.
Microscopic examination of the samples indicated the presence of the filamentous microorganisms in a decreasing as shown in Table 2 (filaments rated as few category of abundance, are not listed).

Table 2.
Abundance of filamentous organisms.
Filament abundance never reached values of high density (corresponding to abundant and excessive categories of abundance) and only occasionally values of medium density (very common). Generally, filament abundance was rated as common (filaments observed in all flocs but at low density in a range of 1–5 filaments per floc) that their overall presence was below levels regarded as pathologic (bulking).
Some well-known filamentous microorganisms such as Thiothrix spp., type 021N, and type 0014 were detected in both reactors during the two experimental periods (i.e., before and after dosing sulphide to reactor SBR1). The filamentous organism Beggiatoa spp. only appeared during the second experimental period (Period 2), although it was present in both reactors and rather sporadically. Thus, the presence of this bacterium was not linked to the external dose of sulphide provided to reactor SBR1, but rather to the presence of sulphide in the sewage or to other factors not related to sulphur compounds. In addition, when some filamentous species (Thiothrix spp., type 021N) became prevalent in the bulk, they never reached the threshold that would determine sludge pathologies (bulking).
Microscopic observation of the activated sludge sampled in both reactors showed good quality features also during the second experimental period (Figure 3). In terms of the protocol proposed by Jenkins, the flocs had good morphological structure, good compactness, their shape was irregular, and their size was 150–500 µm. The filamentous bacteria detected, were mainly located within the floc structure. Their influence upon the structure of flocs was judged irrelevant.
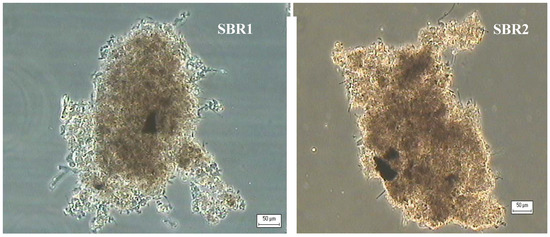
Figure 3.
Floc structure during the second experimental period in the two reactors (100×).
The sludge volume index (SVI) was mostly below 150 mL gMLSS−1 for both reactors, with no substantial differences between SBR1 and SBR2 (Figure 4). During the last 20 days of the experimental period the SVI had a sensible increase which appears related to the rapid increase of temperature [16] in the reactors (warm season), as shown in Figure 4.
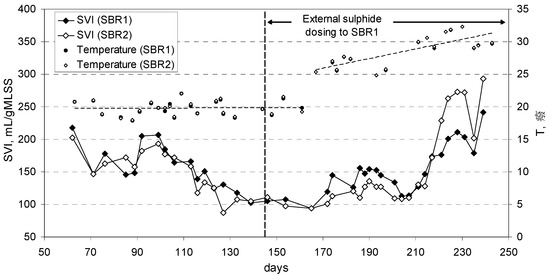
Figure 4.
Sludge volume index and temperature trend in the reactors.
Therefore, microscopic observation showed no relevant differences in both composition and structure of the biomass in the two mixed sludges, during all the period of the experimentation. In particular, the reactor kept under selective pressure of H2S did not show any tendency of the biomass to develop specific filamentous organisms as consequence of the addition of hydrogen sulphide.
4. Conclusions
An experimental test was performed to evaluate possible effects of H2S on activated sludge biomass and biodegradation processes in the Activated Sludge Diffusion system. Under the operating conditions adopted, the following observations were made:
- No significant effects of sulphide on the oxidation ability of the heterotrophs were detected, therefore no effects on COD removal efficiencies.
- No evident effects on nitrification and denitrification due to the presence of sulphide were shown by heterotrophic and autotrophic populations.
- The external sulphide addition to SBR1 did not cause significant differences between the liquid and gaseous effluent sulphide concentrations observed in the two reactors, suggesting that the external dose provided to SBR1 was completely depleted.
- No tendency of the biomass to develop specific organisms was observed as a consequence of the addition of sulphide. Thus no relevant effects of sulphur compounds were observed with respect to sludge pathologies that could affect process efficiencies also in terms of settleability.
Therefore, “activated sludge diffusion” was confirmed as an interesting system among the strategies for odour reduction that can be implemented at wastewater treatment plants [17].
References
- Brock, T.D.; Madigan, M.T. Biology of Microorganism, 5th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Barbosa, V.L.; Burgess, J.E.; Darke, K.; Stuetz, R.M. Activated sludge biotreatment of sulphurous waste emissions. Rev. Environ. Sci. Biotechnol. 2002, 1, 345–362. [Google Scholar] [CrossRef]
- Barbosa, V.L.; Dufol, D.; Callan, J.L.; Sneath, R.; Stuetz, R.M. Hydrogen sulphide removal by activated sludge diffusion. Water Sci. Technol. 2004, 50, 199–205. [Google Scholar] [CrossRef]
- Blonda, M.; Laera, G.; Notarangelo, G.; Palumbo, R.; Pollice, A. Activate Sludge Diffusion for Odour Removal: Effects on the Biomass. In Proceedings of the atti del Simposio Internazionale di Ingegneria Sanitaria Ambientale, Taormina, Italy, 23–26 June 2004. [Google Scholar]
- Bowker, P.G.; Burgess, E. Activated sludge diffusion as an odour control technique. In Odours in Wastewater Treatment. Measurements, Modelling and Control; Stuetz, R., Frechen, F.B., Eds.; IWA Publishing: London, UK, 2001; pp. 415–437. ISBN 1 900222 46 9. [Google Scholar]
- Palumbo, R.; Giorgio, E.; Blonda, M.; Pollice, A.; Laera, G. Microbiological and biochemical effects of activated sludge diffusion for sulphide biooxidation in wastewater treatment plants. In Proceedings of the Congress on Biotechniques for Air Pollution Control, A Coruña, Spain, 5–7 October 2005; pp. 111–118. [Google Scholar]
- Lebrero, R.; Rodriguez, E.; Garcia-Encina, P.A.; Munoz, R. A comparative assessment of biofiltration and activated sludge diffusion for odour abatement. J. Hazard. Mater. 2011, 190, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.L.; Hobbs, P.; Sneath, R.; Burgess, J.; Callan, J.; Stuetz, R. Investigating the capacity of an activated sludge process to reduce volatile organic compounds and odor emissions. Water Environ. Res. 2006, 78, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.L.; Stuetz, R.M. Performance of activated sludge diffusion for biological treatment of hydrogen sulphide gas emissions. Water Sci. Technol. 2013, 68, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Quaderni CNR-IRSA. Il Problema del Bulking Filamentoso e Delle Schiume Biologiche Negli Impianti a Fanghi Attivi; Quaderni Consiglio Nazionale delle Ricerche: Roma, Italy, 1999. [Google Scholar]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking and Foaming, 2nd ed.; Lewis Pubblishers: New York, NY, USA, 1993. [Google Scholar]
- Turk, A.; Bandoz, T. Adsorption Systems for odour treatment. In Odours in Wastewater Treatment. Measurements, Modelling and Control; Stuetz, R., Frechen, F.B., Eds.; IWA Publishing: London, UK, 2001; pp. 345–364. ISBN 1 900222 46 9. [Google Scholar]
- Hansen, N.G.; Rindel, K. Bioscrubbing, An effective and economic solution to odour control at wastewater treatment plants. Water Sci Technol. 2000, 41, 155–164. [Google Scholar] [CrossRef]
- APAT-IRSA-CNR Metodi Analitici per le Acque, Manuali e Linee Guida 29; Agenzia per la Protezione dell’Ambiente e per i Servizi Tecnologici, Istituto di Ricerca Sulle Acque del Consiglio Nazionale Delle Ricerche: Roma, Italy, 2003.
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association (APHA): Washington, DC, USA, 1995.5. [Google Scholar]
- Krishna, C.; van Loosdrecht, M.C.M. Effect of temperature on storage polymers and settleability of activated sludge. Water Res. 1999, 33, 2374–2382. [Google Scholar] [CrossRef]
- Bouzalakos, S.; Jefferson, B.; Longhurst, P.J.; Stuetz, R.N. Developing methods to evaluate odour control products. In Proceedings of the 2nd IWA International Workshop & Conference on Odour and VOCs: Measurement Regulation and Control Techniques, Singapore, 14–17 September 2003; pp. 1–8. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).