Application of Waste Polymers as a Raw Material for Ultrafiltration Membrane Preparation †
Abstract
:1. Introduction
2. Materials and methods
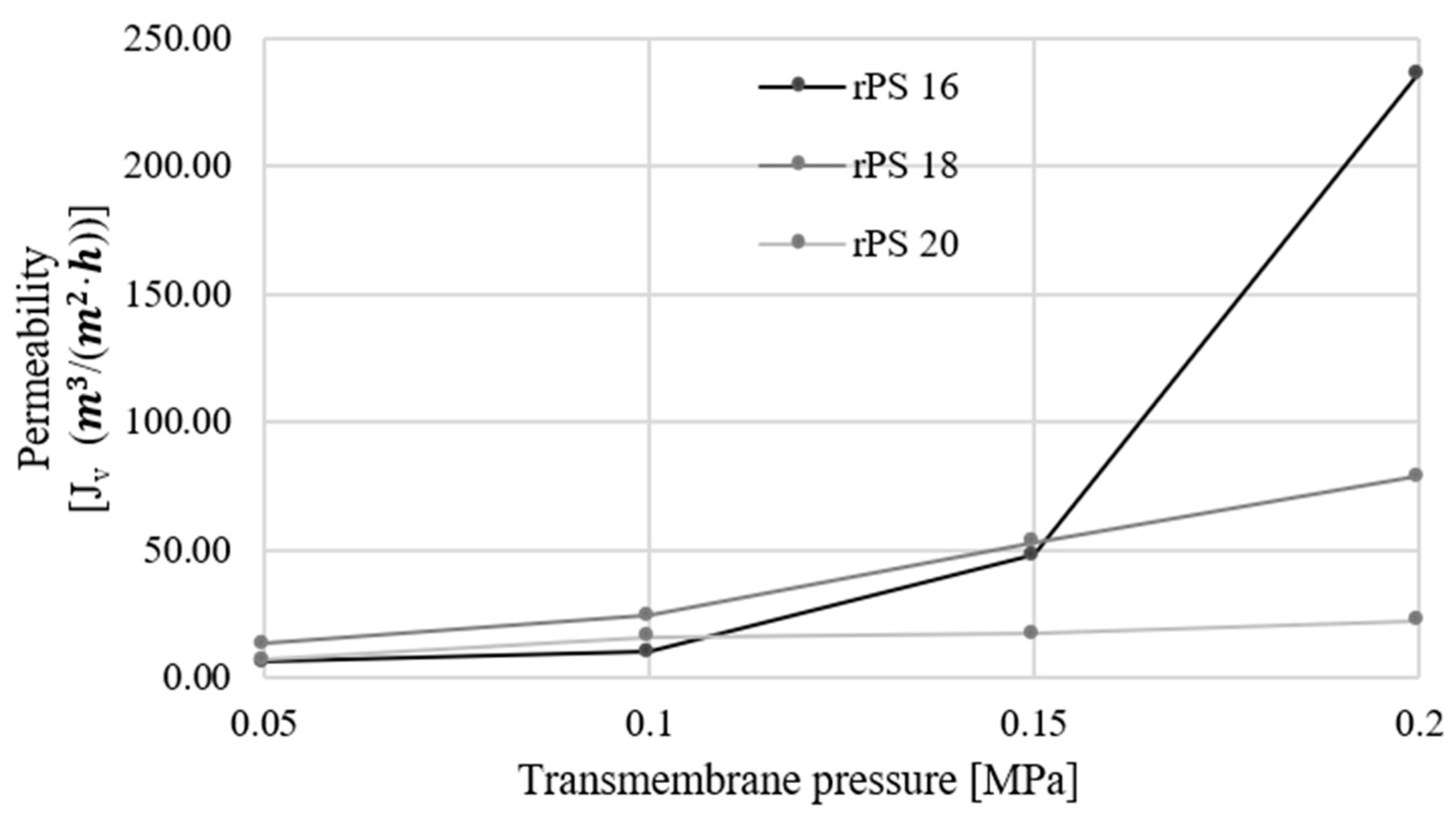
3. Results
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Truchan, K.; Wyszyńska, E. Styropiany nie muszą zaśmiecać. Przegl. Bud. 2008, 12, 49–52. [Google Scholar]
- Chen, Z.; Rana, D.; Matsuura, T.; Yang, Y.; Lan, C.Q. Study on the structure and vacuum membrane distillation performance of PVDF composite membranes: I. Influence of blending. Sep. Purif. Technol. 2014, 133, 303–312. [Google Scholar] [CrossRef]
- Ramos-Olmos, R.; Rogel-Hernandez, E.; Flores-López, L.; Lin, S.W.; Espinoza-Gómez, H. Synthesis and characterization of asymmetric ultrafiltration membrane made with recycled polystyrene foam and different additives. J. Chil. Chem. Soc. 2008, 53, 1705–1708. [Google Scholar] [CrossRef]
- Tiron, L.G.; Pintile, S.C.; Lazar, A.L.; Vlad, M.; Balta, S.; Bodor, M. Influence of Polymer Concentration on Membrane Performance in Wastewater Treatment. Mater. Plast. (Bucharest, Rom.) 2018, 55, 95–98. [Google Scholar] [CrossRef]
- Ratajczak, P. Procesy membranowe—Wprowadzenie. Tech. Wod. 2013, 4, 16–20. [Google Scholar]
- Kliber, S.; Wiśniewski, J. Membranowy proces wymiany anionów jako metoda zmiany składu jonowego wody. Rocz. Ochr. Śr. 2009, 11, 995–1005. [Google Scholar]
- Adamczak, M.; Kamińska, G.; Bohdziewicz, J. The effect of various conditions on the ultrafiltration process of bisphenol A using nanocomposite membranes modified with carbon nanotubes. E3S Web. Conf. 2018, 44, 1–7. [Google Scholar] [CrossRef]
| Membrane Type | Porosity (%) | Contact Angle (%) |
|---|---|---|
| rPS 16 | 38.52 ± 2.27 | 53.30 ± 3.98 |
| rPS 18 | 62.47 ± 3.11 | 49.60 ± 4.86 |
| rPS 20 | 57.10 ± 5.31 | 58.50 ± 5.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczak, M.; Kamińska, G.; Bohdziewicz, J. Application of Waste Polymers as a Raw Material for Ultrafiltration Membrane Preparation. Proceedings 2019, 16, 14. https://doi.org/10.3390/proceedings2019016014
Adamczak M, Kamińska G, Bohdziewicz J. Application of Waste Polymers as a Raw Material for Ultrafiltration Membrane Preparation. Proceedings. 2019; 16(1):14. https://doi.org/10.3390/proceedings2019016014
Chicago/Turabian StyleAdamczak, Michał, Gabriela Kamińska, and Jolanta Bohdziewicz. 2019. "Application of Waste Polymers as a Raw Material for Ultrafiltration Membrane Preparation" Proceedings 16, no. 1: 14. https://doi.org/10.3390/proceedings2019016014
APA StyleAdamczak, M., Kamińska, G., & Bohdziewicz, J. (2019). Application of Waste Polymers as a Raw Material for Ultrafiltration Membrane Preparation. Proceedings, 16(1), 14. https://doi.org/10.3390/proceedings2019016014





