P3HT Processing Study for In-Liquid EGOFET Biosensors: Effects of the Solvent and the Surface †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Device Fabrication
2.3. Characterizations
3. Results and Discussion
3.1. Tapping Mode AFM
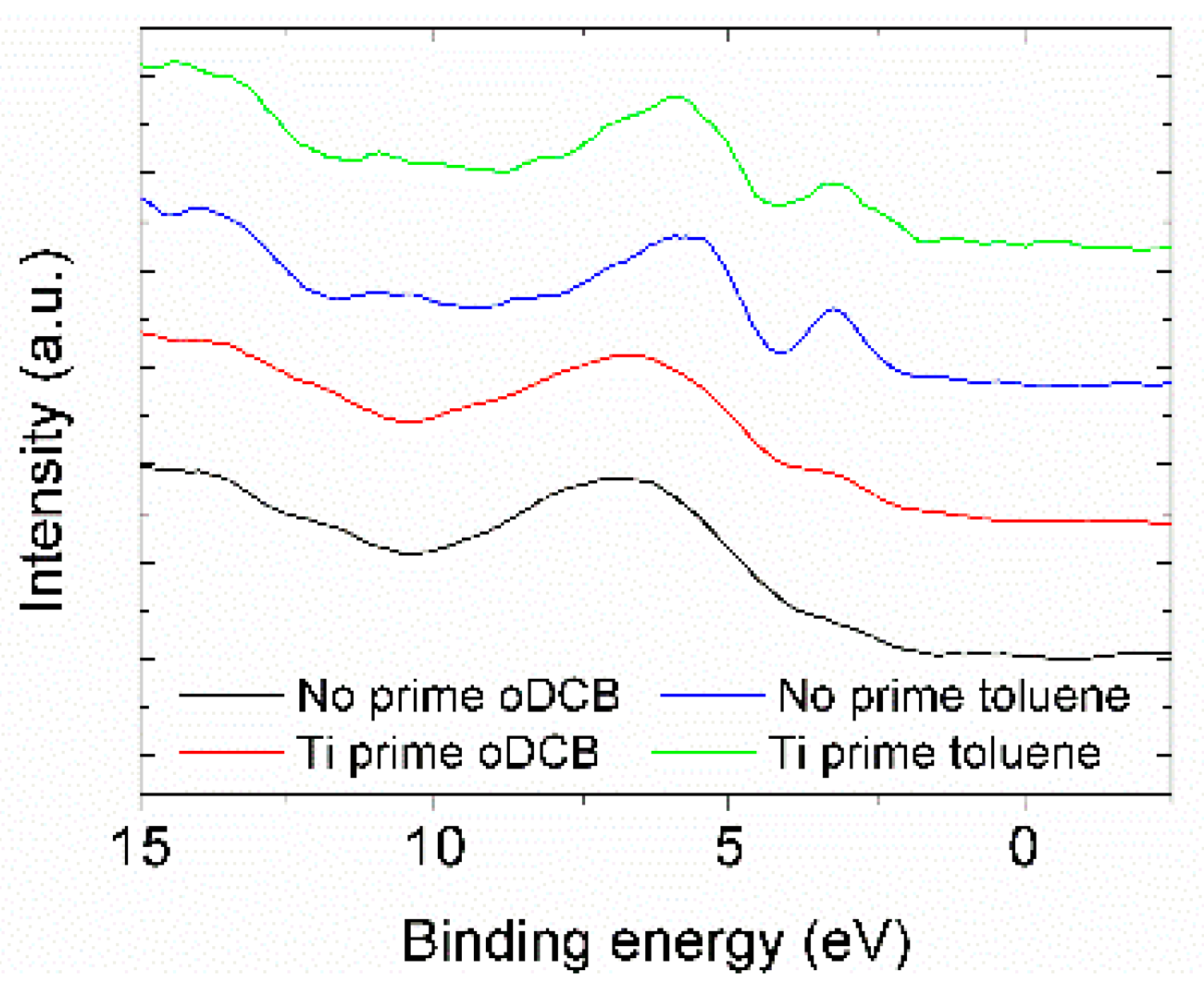
3.2. XPS Characterization
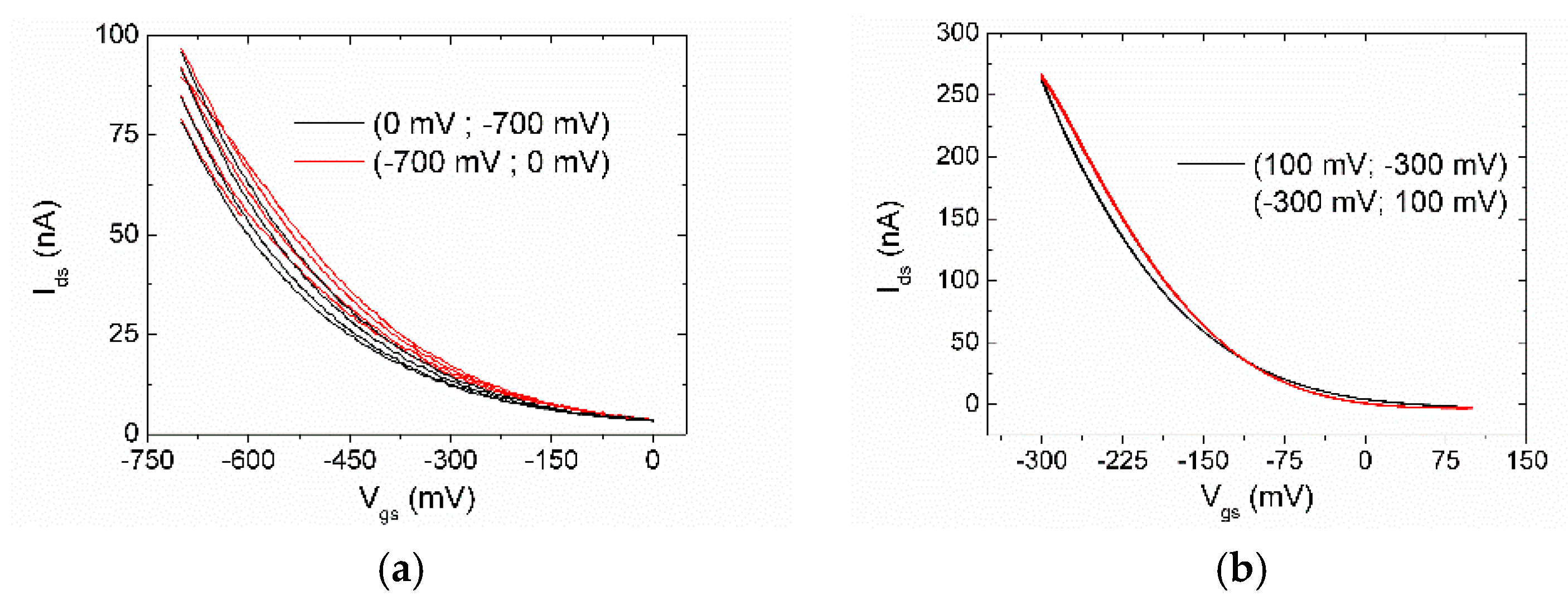
3.3. Electrical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kergoat, L.; Piro, B.; Berggren, M. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Ludwigs, S. P3HT Revisited—From Molecular Scale to Solar Cell Devices. Adv. Polym. Sci. 2014, 265, 39–82. [Google Scholar]
- Seshadri, P.; Manoli, K.; Schneiderhan-marra, N.; Anthes, U.; Wierzchowiec, P.; Bonrad, K.; Di, C.; Torsi, L. Label-free procalcitonin analytical detection with an electrolyte-gated organic field-effect transistor based electronic immunosensor. Biosens. Bioelectron. 2018, 104, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, B.H. Device Physics of Solution-Processed Organic Field-Effect Transistors. Adv. Mat. 2005, 17, 2411–2425. [Google Scholar] [CrossRef]
- D’Angelo, P.; Tarabella, G.; Romeo, A.; Giodice, A.; Marasso, S.; Cocuzza, M.; Ravanetti, F.; Cacchioli, A.; Petronini, P.G.; Iannotta, S. Monitoring the adaptive cell response to hyperosmotic stress by organic devices. Mrs Commun. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Angelo, P.D.; Tarabella, G.; Romeo, A.; Marasso, S.L.; Verna, A.; Cocuzza, M.; Peruzzi, C.; Vurro, D.; Iannotta, S.; D’Angelo, P.; et al. PEDOT:PSS Morphostructure and Ion-To-Electron Transduction and Amplification Mechanisms in Organic Electrochemical Transistors. Materials 2018, 12, 9. [Google Scholar] [PubMed]
- Dannetun, P.; Boman, M.; Stafström, S.; Salaneck, W.R.; Lazzaroni, R.; Fredriksson, C.; Brédas, J.L.; Taliani, C.; Dannetun, P.; Boman, M.; et al. The chemical and electronic structure of the interface between aluminum and polythiophene semiconductors. J. Chem. Phys. 1993, 99, 664–671. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmeggiani, M.; Verna, A.; Ballesio, A.; Cocuzza, M.; Piatti, E.; Fra, V.; Pirri, C.F.; Marasso, S.L. P3HT Processing Study for In-Liquid EGOFET Biosensors: Effects of the Solvent and the Surface. Proceedings 2019, 15, 39. https://doi.org/10.3390/proceedings2019015039
Parmeggiani M, Verna A, Ballesio A, Cocuzza M, Piatti E, Fra V, Pirri CF, Marasso SL. P3HT Processing Study for In-Liquid EGOFET Biosensors: Effects of the Solvent and the Surface. Proceedings. 2019; 15(1):39. https://doi.org/10.3390/proceedings2019015039
Chicago/Turabian StyleParmeggiani, Matteo, Alessio Verna, Alberto Ballesio, Matteo Cocuzza, Erik Piatti, Vittorio Fra, Candido Fabrizio Pirri, and Simone Luigi Marasso. 2019. "P3HT Processing Study for In-Liquid EGOFET Biosensors: Effects of the Solvent and the Surface" Proceedings 15, no. 1: 39. https://doi.org/10.3390/proceedings2019015039
APA StyleParmeggiani, M., Verna, A., Ballesio, A., Cocuzza, M., Piatti, E., Fra, V., Pirri, C. F., & Marasso, S. L. (2019). P3HT Processing Study for In-Liquid EGOFET Biosensors: Effects of the Solvent and the Surface. Proceedings, 15(1), 39. https://doi.org/10.3390/proceedings2019015039






