Introduction
Formaldehyde HCOH is a toxic compound that, in trace concentrations, causes serious diseases of the respiratory tract, gastrointestinal tract and eyes. The biochemical oxidation of HCOH in human tissues occurs with the formation of CO2 and formic acid, which with prolonged exposure is the cause of asthma, pulmonary edema, and cancer. The main problem in the HCOH detection is the need to determine very low concentrations: 0.5 mg/m3 (0.4 ppm) and 0.1 mg/m3 (81 ppb) in the air of the working and living area, respectively. A promising alternative for the HCOH quantification is the development of gas analyzers based on an array of metal oxide semiconductor sensors that exhibit the specific temperature dependence of the sensor signal when detecting different gases. It is currently shown that the use of such sensor arrays allows the detection of trace concentrations of individual substances, including various VOCs, in gas mixtures, as well as the recognition of gas mixture components. Such an analysis is relevant not only for environmental control and safety systems, but also promising for the development of new express methods for analyzing food quality, water purity, and medical diagnostics. However, the use of a dynamic temperature regime implies cyclic heating up to 500 °C, which leads to a significant increase in energy consumption and degradation of the sensitive layer. An alternative to dynamic thermal heating can be periodic photoactivation by UV or visible light with subsequent analysis of the photoconductivity rise and fall curves using a mathematical algorithm. This becomes possible when nanocomposites based on nanocrystalline semiconductor metal oxides and photocatalysts providing low-temperature decomposition/oxidation of target gas molecules are used as sensitive materials.
Experimental
Nanocomposites SnO2/TiO2 modified with Pt, Ag and Au nanoparticles were obtained by wet chemical synthesis. SnO2 xH2O xerogel was precipitated from H2SnCl6 solution. To obtain the SnO2/TiO2 composites, the SnO2 xH2O xerogel and Ti(OPr)4 alcohol solution were stirred at RT till full hydrolysis of titanium precursor. The resulting solid phase was impregnated with Pt(acac)2, AgNO3 and previously formed Au sol and then annealed at 300 °C for 24 h. The composition and microstructure of the samples were characterized by EDX, XRD, HRTEM and single-point BET methods. The surface sites were investigated using thermal analysis, FTIR and XPS. The sensor measurements were carried out in the temperature range 50–300 °C in the flow cell shielded from the background light. DC measurements were carried out to monitor the electrical conductance of the sample during exposure to HCOH/air gas mixtures (0.06–0.6 ppm HCOH in dry air) in dark conditions and under constant and periodic lighting. Miniature LEDs with λmax = 365 nm (UV), λmax = 470 nm (blue), λmax = 535 nm (green) and λmax = 630 nm (red) inserted into the cell were used as illumination sources.
Results
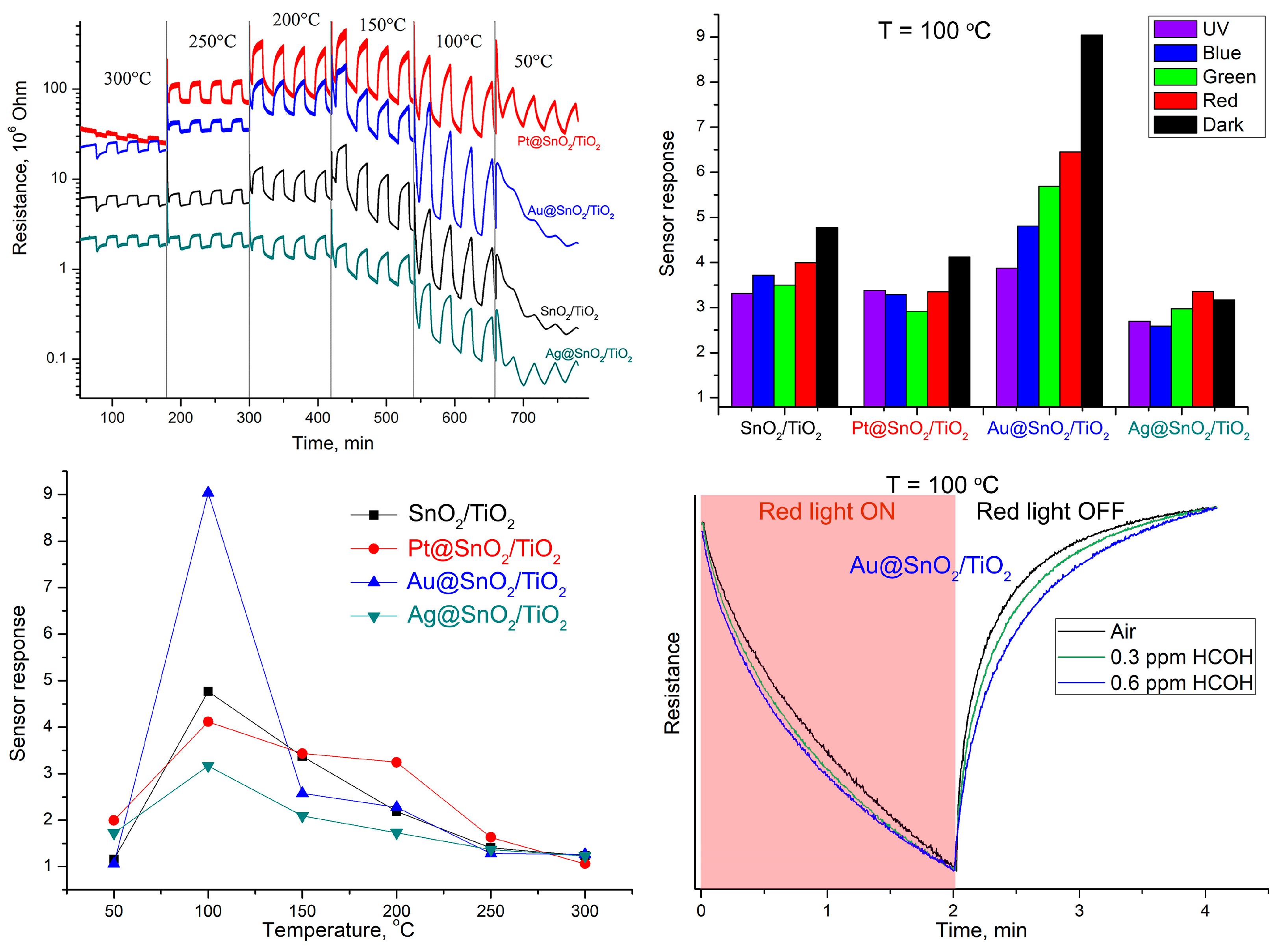
Figure 1a shows the change in the samples resistance at periodic changing the composition of the gas phase: “dry air—0.6 ppm HCHO in dry air”, in the temperature range 300–50 °C in dark conditions. From the obtained data the values of the sensor response S were calculated as S = Rair/Rgas, where Rair and Rgas are the values of the nanocomposite resistance in pure air and in the presence of HCHO, respectively (
Figure 1b). The best sensor response in dark conditions was obtained for Au@SnO2/TiO2 sample at 100 °C. The constant illumination of the samples during the measurements at 100 °C leads to a decrease in the sensor response (
Figure 1c), and this decrease in the signal value growths with increasing radiation energy from red to UV light. However, the use of periodic illumination (
Figure 1d) makes it possible to obtain the dependences of photoconductivity on the concentration of HCOH for their further description using a mathematical signal processing algorithm. Such a replacement of the dynamic temperature regime with periodic illumination will allow the quantitative determination of HCOH in gas mixtures with a significant reduction in energy consumption.
Funding
This research was funded by the Russian Ministry of Education and Sciences (Agreement No. 14.613.21.0075, RFMEFI61317X0075).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).