Consideration for Oxygen Adsorption Species on SnO2 Semiconductor Gas Sensors †
1. Introduction
2. Experiments
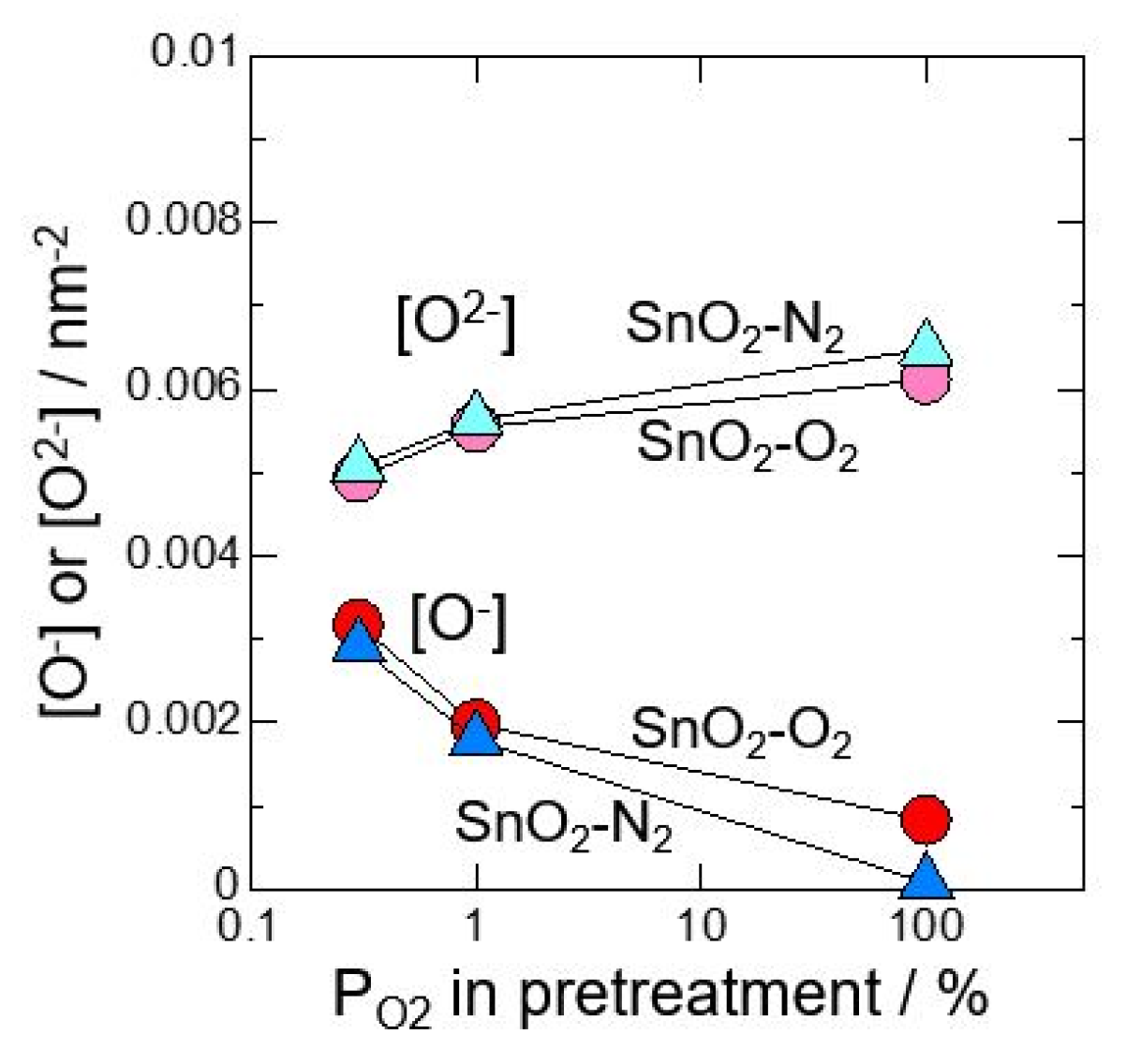
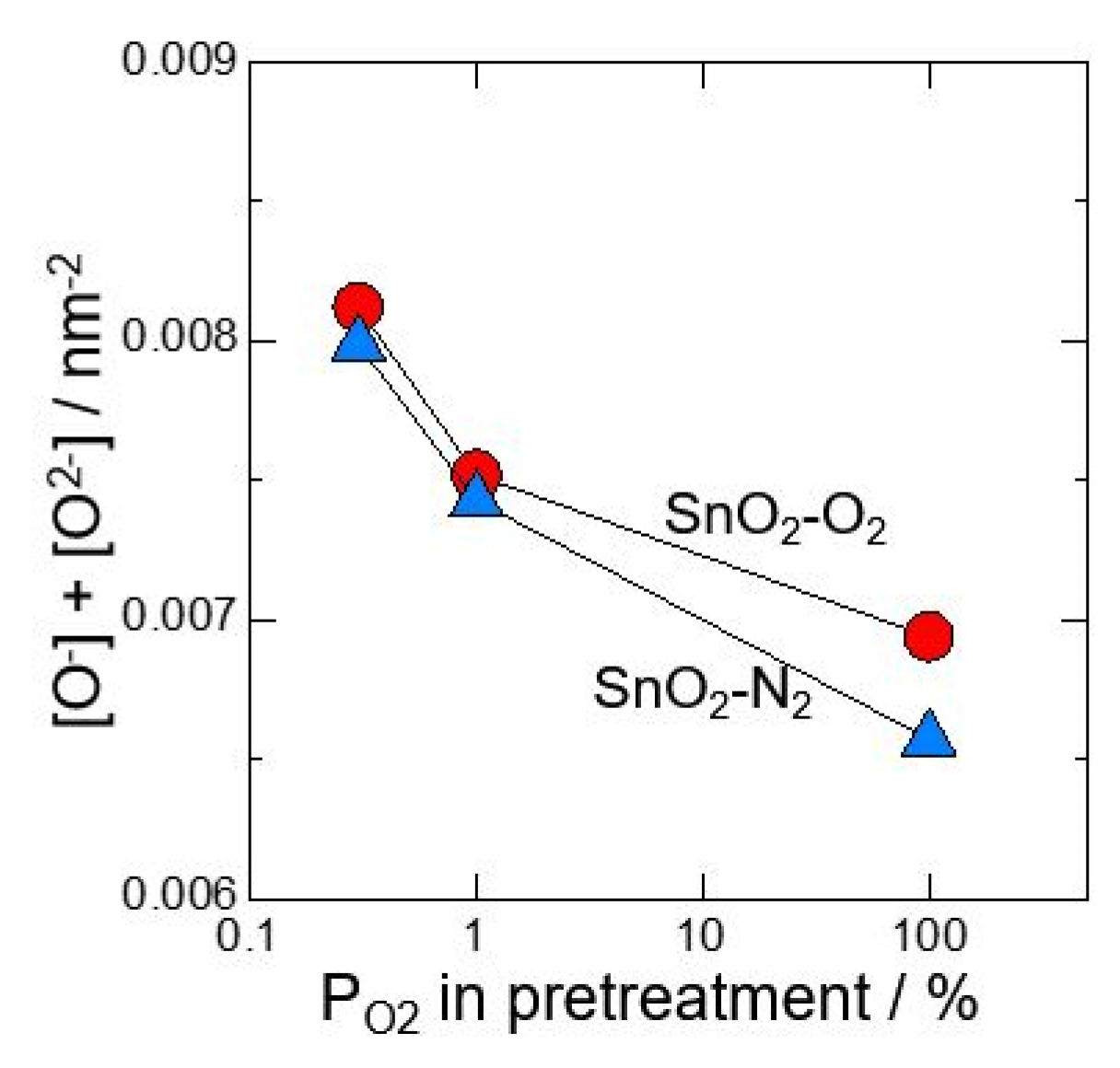
3. Results and Discussion
Reference
- Yamazoe, N.; Suematsu, K.; Shimanoe, K. Extension of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens. Actuators B Chem. 2012, 163, 128–135. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimanoe, K.; Mizukami, T.; Suematsu, K.; Watanabe, K. Consideration for Oxygen Adsorption Species on SnO2 Semiconductor Gas Sensors. Proceedings 2019, 14, 18. https://doi.org/10.3390/proceedings2019014018
Shimanoe K, Mizukami T, Suematsu K, Watanabe K. Consideration for Oxygen Adsorption Species on SnO2 Semiconductor Gas Sensors. Proceedings. 2019; 14(1):18. https://doi.org/10.3390/proceedings2019014018
Chicago/Turabian StyleShimanoe, Kengo, Takaharu Mizukami, Koichi Suematsu, and Ken Watanabe. 2019. "Consideration for Oxygen Adsorption Species on SnO2 Semiconductor Gas Sensors" Proceedings 14, no. 1: 18. https://doi.org/10.3390/proceedings2019014018
APA StyleShimanoe, K., Mizukami, T., Suematsu, K., & Watanabe, K. (2019). Consideration for Oxygen Adsorption Species on SnO2 Semiconductor Gas Sensors. Proceedings, 14(1), 18. https://doi.org/10.3390/proceedings2019014018



