Chemical Equitable Partitions: A New Perspective on Molecular Symmetries and Its Implications for the Study of Structure–Property Relationships †
Abstract
1. Introduction
2. Materials and Methods
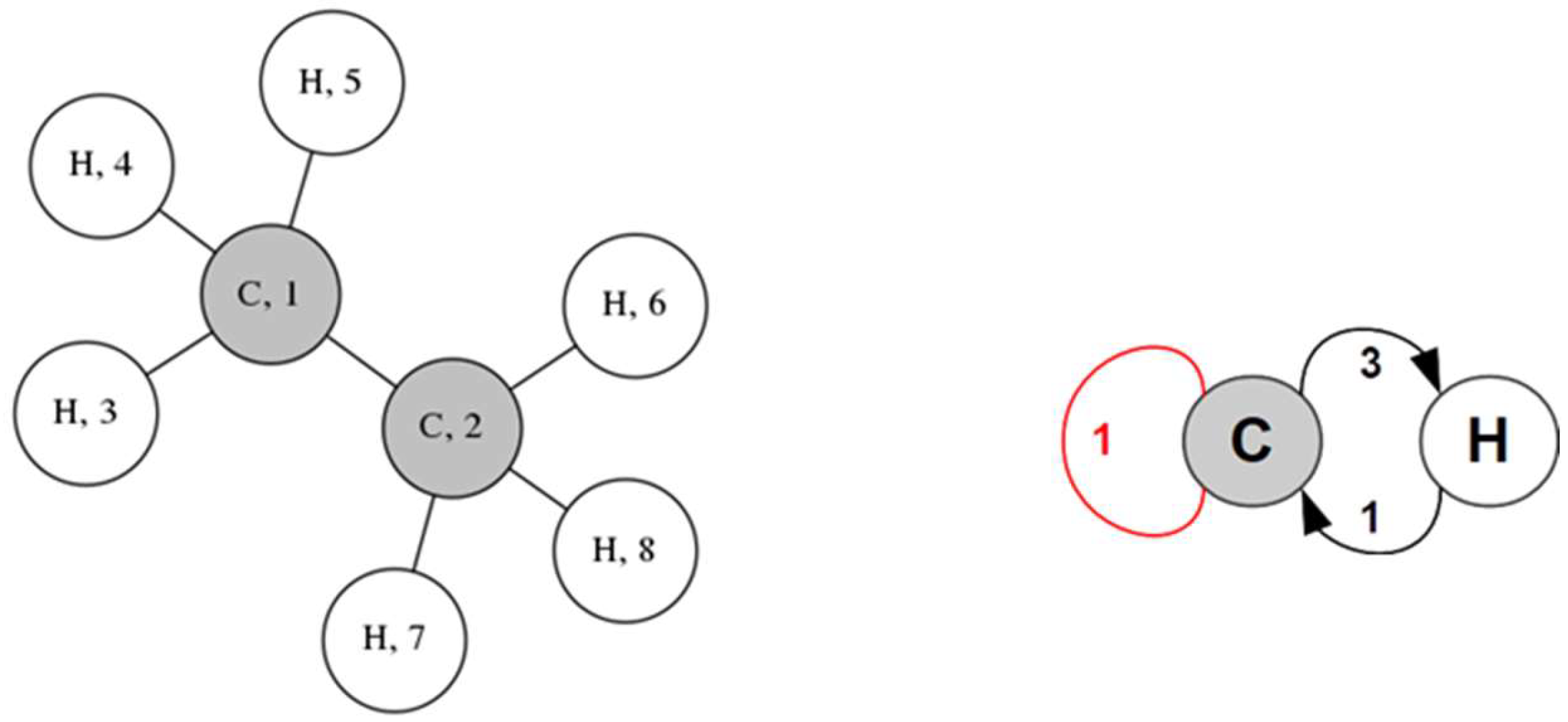
- Initialization: The CP of the molecule is defined according to the chemical identity and degree of the graph’s vertices.
- Classification: Vertices are categorized according to their connectivity profiles of the most recent partition, P, resulting in a new partition, P’.
- Check for equality: P and P’ are compared.
- ο
- If not identical: Replace P by P’ and go back to the classification step.
- ο
- If identical: Terminate.
3. Results
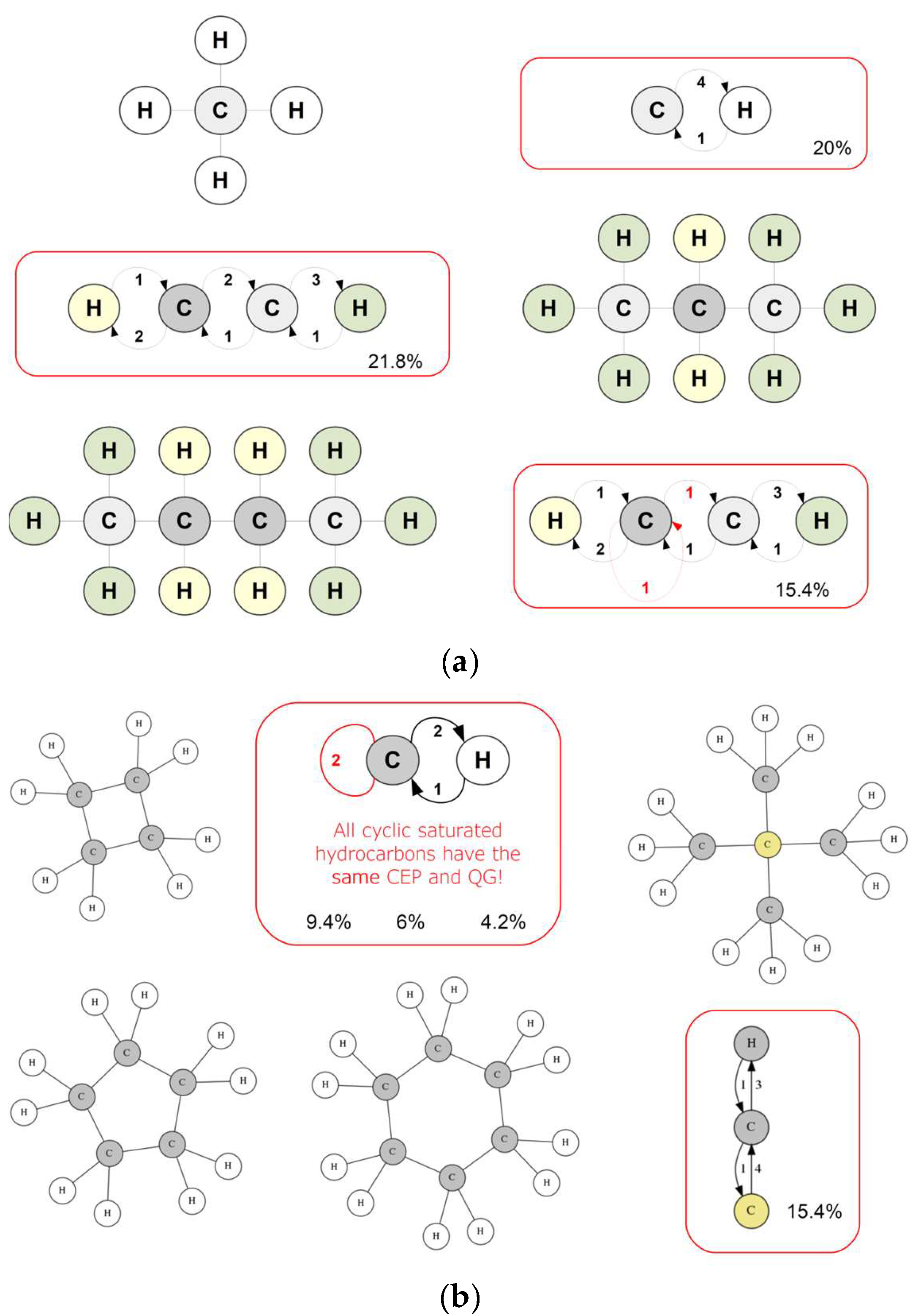
3.1. Demonstration of Foundational Concepts
- Linear alkanes present the most complex QGs as atoms are differentiated relative to their distance from the chain ends; notably, there is at most one loop in the QG, depending on whether there is an even or an odd number of carbons.
- Ring alkanes share the same very simple QG, which leads to a decreasing CR with molecular size, reflecting a correspondingly higher symmetry.
- The example of neopentane showcases the effect of symmetry in the form of a small and simple QG relative to a large molecule.
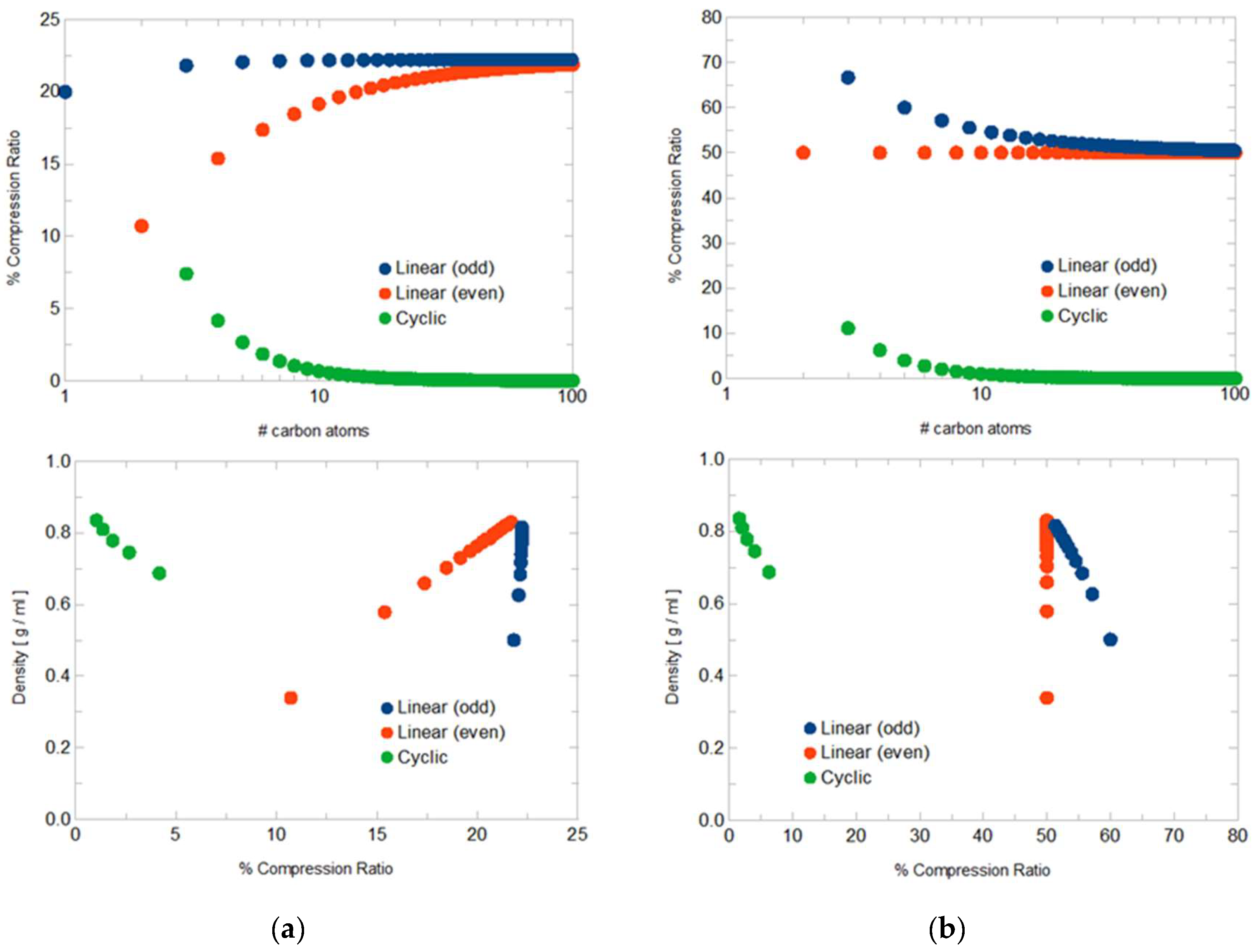
3.2. Molecular Graph Symmetry and Physical Properties
- Cyclic alkanes exhibit a compression ratio tending towards zero in molecular size, as expected, and a relatively clear correlation between CR and mass density.
- United-atom graphs result in higher CR values. The explicit presence of hydrogen atoms implies more information content to compress; omission of hydrogen atoms means that the graphs are already ‘half-compressed’.
- The homologous series of linear alkanes is split into two categories with different correlation between CR and molecular size, or CR and mass density, depending on the even or odd number of carbon atoms. This is due to the presence or not of a loop in the quotient graph, as mentioned in Section 3.1.
3.3. Extension to Complex Topologies
- Cyclic alkanes in their united-atom form have zero information content.
- The addition of an alkyl tail to a ring molecule breaks its symmetry; this is clearly quantified for each molecule by increasing values of CR.
- CycYY families form smooth curves (shown with green colour in the figure) directed roughly from bottom left to top right (except Cyc00), indicating positive correlation of their CR and IG (increasing information content with increasing asymmetry).
- Cyc00 and AlkYY families form zig-zag lines (shown with dark grey colour); the envelope curves of these lines (denoted by alternating red and blue colour) generally exhibit an anticorrelation between CR and IG, which becomes more prominent for values of IG around 2 to 4; and CR around 100 to 150%.
- Linear alkanes and all other alkylcycloalkanes feature an even/odd distinction regarding the number of their carbon atoms, with red and blue envelop lines corresponding to even and odd numbers, respectively. This observation generalises the finding of even-odd distinction in the case of linear alkanes, as discussed in Section 3.2.
- ‘Iso-alkyl’ and ‘iso-ring’ curves are clearly formed by the envelope curves corresponding to different fixed sizes of alkyl tails, and the curves of fixed-size cycloalkyl rings, respectively. These curves, which define a set of ‘chemical coordinates’, are smooth enough to be parameterized as transformations of the (CR, IG) plane.
- Several branched alkanes tend to cluster parallel to the iso-ring curves. Therefore, it should be possible to model complex molecules using real-valued ‘chemical coordinates’, with the physical significance of fractional contributions of building blocks, e.g., alkyl and cycloalkyl groups, of simpler molecules with known properties.
4. Discussion
- Investigating correlations of more physical properties with graph-theoretic measures, as discussed in Section 3, for larger families of organic compounds.
- Enhancing machine learning schemes for the prediction of physical properties of compounds, by the addition of the above-defined graph theoretic measures and variants.
- Adjusting the above-mentioned methods to inorganic and hybrid organic–inorganic lattices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Chemical Partition |
| cEP | Coarsest Equitable Partition |
| CEP | Chemical Equitable Partition |
| CR | Compression Ratio |
| EP | Equitable Partition |
| QG | Quotient Graph |
References
- Biggs, N. Algebraic Graph Theory; Cambridge University Press: New York, NY, USA, 1974. [Google Scholar]
- Cvetkovic, D.; Doob, M.; Sachs, H. Spectra of Graphs: Theory & Application (Volume 87 of Pure and Applied Mathematics); Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Wagner, S.; Wang, H. Introduction to Chemical Graph Theory; Taylor & Francis: New York, NY, USA, 2018. [Google Scholar]
- Michos, I.; Raptis, V. Graph Partitions in Chemistry. Entropy 2023, 25, 1504. [Google Scholar] [CrossRef] [PubMed]
- Raptis, V.; Kaltzoglou, A. Graph-theoretical Analysis as an Aid in the Elucidation of Structure-Property Relations of Perovskite Materials. AIP Conf. Proc. 2024, 3030, 110005. [Google Scholar]
- Godsil, C.; Royle, G.F. Algebraic Graph Theory; Springer: New York, NY, USA, 2001. [Google Scholar]
- McKay, B.D.; Piperno, A. Practical graph isomorphism, II. J. Symb. Comput. 2014, 60, 94–112. [Google Scholar] [CrossRef]
- Hajiabolhassan, H.; Taheri, Z.; Hojatnia, A.; Yeganeh, Y.T. FunQG: Molecular Representation Learning via Quotient Graphs. J. Chem. Inf. Model. 2023, 63, 3275–3287. [Google Scholar] [CrossRef] [PubMed]
- Weisfeiler, B.; Leman, A. The reduction of a graph to canonical form and the algebra which appears therein. Nauchno-Tech. Informatsia Ser. 2 1968, 9, 12–16. [Google Scholar]
- Corneil, D.G.; Cotlieb, C.C. An efficient algorithm for graph isomorphism. J. Assoc. Comput. Mach. 1970, 17, 51–64. [Google Scholar] [CrossRef]
- McKay, B.D. Computing automorphisms and canonical labellings of graphs. In the Combinatorial Mathematics. Lecture Notes in Mathematics; Springer: Berlin/Heidelberg, Germany, 1978; Volume 686, pp. 223–232. [Google Scholar]
- Cao, M.; Zhang, S.; Camlibel, M. A Class of Uncontrollable Diffusively Coupled Multiagent Systems with Multichain Topologies. IEEE Trans. Autom. Control 2013, 58, 465. [Google Scholar] [CrossRef]
- O’ Clery, N.; Yuan, Y.; Stan, G.-B.; Barahona, M. Observability and coarse graining of consensus dynamics through the external equitable partition. Phys. Rev. E 2013, 88, 042805. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S. Topological complexity of crystal structures: Quantitative approach. Acta Cryst. 2012, A68, 393. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, W.A.; Cholakov, G.S.; Stateva, R.P. Liquid Density and Critical Properties of Hydrocarbons Estimated from Molecular Structure. J. Chem. Eng. Data 2002, 47, 559–570. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Van Krevelen, D.W. Properties of Polymers, 3rd ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raptis, V.; Michos, I. Chemical Equitable Partitions: A New Perspective on Molecular Symmetries and Its Implications for the Study of Structure–Property Relationships. Proceedings 2025, 123, 6. https://doi.org/10.3390/proceedings2025123006
Raptis V, Michos I. Chemical Equitable Partitions: A New Perspective on Molecular Symmetries and Its Implications for the Study of Structure–Property Relationships. Proceedings. 2025; 123(1):6. https://doi.org/10.3390/proceedings2025123006
Chicago/Turabian StyleRaptis, Vasilios, and Ioannis Michos. 2025. "Chemical Equitable Partitions: A New Perspective on Molecular Symmetries and Its Implications for the Study of Structure–Property Relationships" Proceedings 123, no. 1: 6. https://doi.org/10.3390/proceedings2025123006
APA StyleRaptis, V., & Michos, I. (2025). Chemical Equitable Partitions: A New Perspective on Molecular Symmetries and Its Implications for the Study of Structure–Property Relationships. Proceedings, 123(1), 6. https://doi.org/10.3390/proceedings2025123006






