1. Introduction
Aquaculture is arguably the fastest-growing food production sector, accounting for nearly 52% of global fish consumption and seen as a key solution to meeting the rising demand for aquatic products. It plays a significant role in food security and nutrition in the 21st century [
1]. In Togo, aquaculture contributes only 5.08% of national fish production. However, total fish production—both from aquaculture and capture fisheries—meets just 30% of the country’s domestic output [
2], despite Togo’s natural potential for aquaculture development [
3]. Tilapia farming (genus
Oreochromis) dominates, primarily in semi-intensive systems [
4,
5]. Thus, improving local aquaculture production is critical to reducing dependence on declining marine fisheries and imports.
Accelerating the adoption of sustainable and equitable aquaculture practices requires advancing policies, management, investments, and innovation [
1]. Feed, a critical link accounting for 60–70% of production costs, is a priority area for technological innovation [
6], as it significantly influences fish farm productivity. Fishmeal is the primary protein source in aquafeed [
7,
8], but its rising cost drives the need for alternative protein sources.
Scientific efforts to improve tilapia feed have focused on valorizing agricultural by-products [
3,
4,
5,
9,
10]. However, alternative soybean (
Glycine max) processing methods, such as dormancy breaking through water soaking, remain underexplored. Soybean, rich in digestible proteins, has superior nutritional value compared to cereal proteins [
11,
12], making it the second-most important protein source in fish feed after fishmeal. However, soybeans contain antinutritional compounds like tannins, saponins, phytic acid, protease inhibitors, gossypol, and aflatoxins [
13,
14].
Dormancy-breaking techniques aim to enhance protein bioavailability while reducing antinutritional factors, offering an alternative to roasting, which is cost-intensive and can degrade nutritional quality if poorly managed.
This study aims to improve aquaculture productivity in Togo through alternative soybean processing methods for sustainable fish feed. Specifically, it evaluates the effects of germination and post-germination drying on crude protein content and aflatoxin levels in soybeans. Additionally, it conducts a preliminary assessment of diets incorporating germinated and dried soybeans on the growth performance of Nile tilapia fry (Oreochromis niloticus).
4. Discussion
The environmental conditions under which the fish were raised were consistent across all diets. In all experimental tanks, the values of the water’s physicochemical parameters remained within the optimal range, except for dissolved oxygen levels [
22,
23,
24]. The low dissolved oxygen levels observed can be attributed to the groundwater source used, combined with the absence of artificial aeration devices in the tanks. Nevertheless, these oxygen levels were not influenced by the type of diet. Thus, the fish were exposed to the same environmental conditions, and the differences in their growth performance cannot be attributed to the rearing environment.
Chemical composition analysis revealed that SG1S was richer in protein, followed by ST and SC. The lower protein levels in fresh samples may be explained by the high water content, which acts as a dilution factor. Dormancy breaking during germination affects the chemical composition of seeds by activating existing enzymes and synthesizing new ones, such as proteases for proteins, lipases for lipids, and amylases for starch [
25,
26]. These enzymatic activities likely increased the protein, lipid, and carbohydrate content in SG1S. Rapid drying halts enzymatic processes by swiftly removing water, resulting in a higher concentration of proteins in roasted and dried soybean samples.
In contrast to chemical composition, SG1F exhibited lower aflatoxin levels than SG1S, SC, and ST. This difference may be linked to storage or preservation conditions. According to Ahmed et al. [
27], storing seeds with moisture content above 10% promotes mold proliferation.
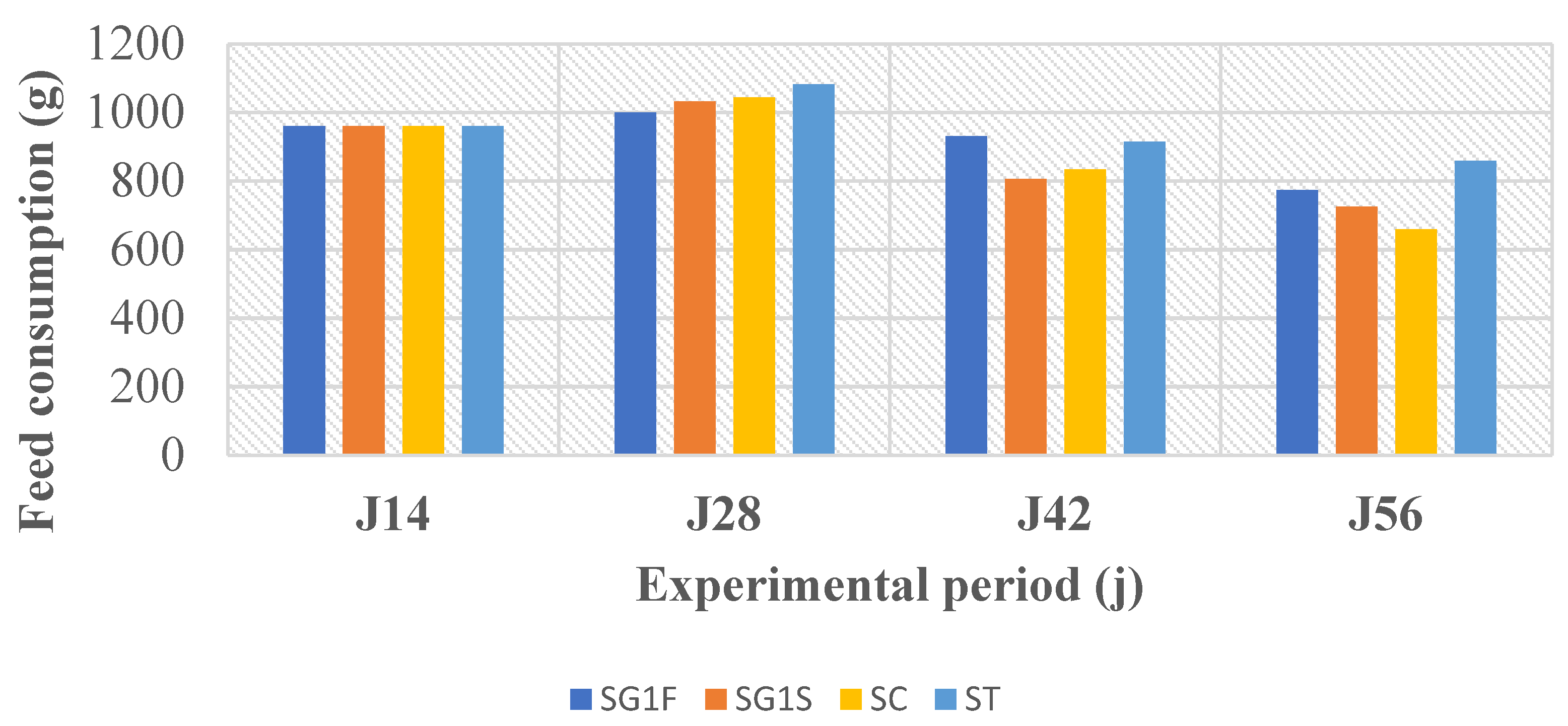
Statistical analyses of feed utilization parameters revealed no significant differences among diets. Thus, at a 5% error margin, it can be concluded that the fish consumed the diets similarly without showing dietary preference.
Regarding feed utilization indices (FCR and FER), the results showed that ST exhibited the best performance (lowest FCR and highest FER), followed by SC, SG1S, and SG1F. These differences are due to the distinct physicochemical characteristics of the soybean treatments.
Growth performance results indicated that the reference diet ST had the best performance, followed by SC, SG1S, and SG1F. Variance analysis revealed a significant effect on weight gain at a 5% threshold (p < 0.05), with ST being significantly superior to other treatments (SC, SG1S, SG1F). Similar trends were observed for weight gain (WG), specific growth rate (SGR), and daily growth rate (DGR), which may be attributed to its higher organic content, including protein (38.42%), lipids (13.13%), and carbohydrates (37.08%).
The weight gain range observed in this study surpassed that reported by Boma et al. [
10] at the same station using blood meal as a protein source over 42 days of feeding, but was lower than the results of Kirimi et al. [
28] in Kenya, who used blood meal over a 100-day feeding period. The specific growth rate (SGR) obtained in this study was lower than that of Dibala et al. [
24] in Burkina Faso, who used plant-based proteins over 56 days of rearing in plastic tanks, but higher than the results of Boma et al. [
10]. The daily growth rate (DGR) was lower than those reported by Dibala et al. [
24].
This study highlights the superiority of the roasted soybean (ST) diet compared to the SG1S diet, despite their theoretically comparable protein levels. Furthermore, our findings indicate that the aflatoxin content in SG1S meals was relatively high compared to ST. Numerous studies have demonstrated the detrimental effects of aflatoxins on fish growth [
14,
29]. This study suggests that optimal preservation of SG1S meals could minimize these factors, improving fish growth when fed this diet.
The survival rates observed during the experiment were high, ranging between 80% and 100%. This indicates that soybeans, in the forms used for this study’s formulations, were non-toxic to fish. The high survival rate of fish fed raw soybean (90.67%), second only to SG1F (100%), demonstrates that incorporating raw soybeans at 30% is not toxic to Nile tilapia. However, our survival rate results exceeded those of Bamba et al. [
30] in Côte d’Ivoire, who reported survival rates between 75% and 95%, but were slightly lower than those of Boma et al. [
10], who reported rates between 96.67% and 100% for a study conducted at the same station in Agbodrafo.