Banded iron formations are Fe- and Si-rich sedimentary rocks deposited from the Eoarchean Era to the Paleoproterozoic Era [1]. Regarding the mineralogy and iron formations, where the total iron content can be up to 40 wt.% [2], the Fe is composed of carbonates, silicates, sulfides, and hydroxide, commonly occurring in variable combinations. The presence of carbonates in Earth’s interior is related to the subduction process, one of the first steps in cycling carbon throughout Earth [3]. Carbonate minerals are important constituents of many rock types and are represented by CaCO3 (calcite), MgCO3 (magnesite), and FeCO3 (siderite). Siderite has attracted considerable attention as this mineral is related to CO2 capture [4,5], a natural protection against corrosion [6,7], a source of iron ore [8,9], and much more. The thermal decomposition of siderite is significant because of the industrial relevance of this mineral [10,11]. The mechanism of this process depends on the siderite composition and experimental conditions like temperature, atmosphere, annealing time, or sample microstructure. The process of the thermal decomposition of siderite [12,13] and the products of this transformation under various external conditions are relatively well described. However, most of the research focuses on the final product of siderite decomposition, and such research is usually conducted in air. In this work, we aim to show not only what the final product of the decomposition of this iron carbonate is but also how exactly this process proceeds as the temperature increases. Moreover, in our research, we pay particular attention to the influence of time on the sider decomposition process and the atmosphere in which the investigated sample is located (air or vacuum).
The siderite high-temperature decomposition and products of this process in air and a vacuum atmosphere were characterized using 57Fe Mössbauer spectroscopy. In this method, the Fe nucleus was treated as a probe of its local surroundings. Based on the results of this method, we obtained information about the iron oxidation states, the iron local microenvironments, the iron magnetic states, and the relative fractions of the iron-bearing components [14]. This study has profound implications for processing siderite ore through metallurgy and ore dressing. The Mössbauer investigation was supported by the X-ray diffraction and X-ray fluorescence methods.
Various carbonate rocks are present in the Upper Silesian Coal Basin, Poland. These are mainly siderites in the form of spherosiderites, shoal siderites, and carbonaceous siderites. The studied siderites come from the Ruda coal seams’ upper parts, in the Chwałowice Trough’s eastern part, in the Upper Silesian Coal Basin. The research was carried out on two rock samples taken from different seams. These samples were designated Sd1 and Sd3. The siderite samples were heated in air and a vacuum from room temperature to 700 °C for one hour. After heating at a given temperature, the Mössbauer measurements and, for selected samples, XRD measurements were performed.
The measurements by XRD revealed that the rock siderite samples contained siderite as the main component and small amounts of accessory minerals. The Sd1 sample contained ~94% siderite and 5% kaolinite. However, the Sd3 sample contained 84% siderite, and the remainder was mainly kaolinite (6%) and quartz (7%). Moreover, the Sd1 sample contained 37% Fe and 1% Mg, and the Sd3 sample contained slightly less, 28% Fe, but much more magnesium, as much as 4%. The only and main phase containing Fe was siderite, which makes 57Fe Mössbauer spectroscopy an excellent tool for studying the processes it undergoes during annealing. Figure 1 shows the selected Mössbauer spectra for the Sd1 sample heated for one hour at defined temperatures, in air and in a vacuum.
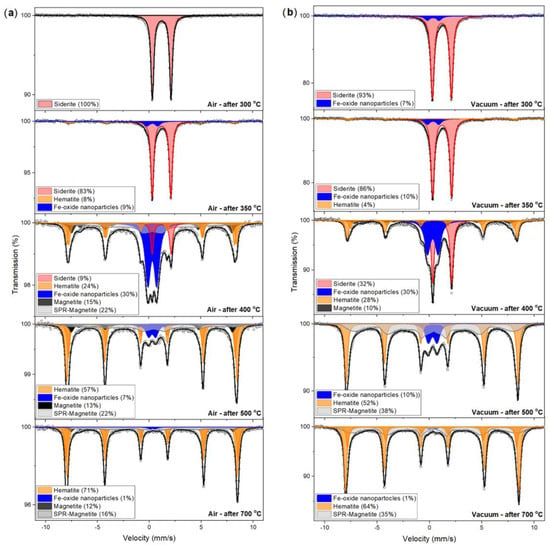
Figure 1.
57Fe Mössbauer spectra of siderite sample Sd1 heated at different temperatures in the air (a) and vacuum (b) atmosphere for one hour. Fitted subspectra (colored lines), their phase assignment, and contributions are shown on each spectrum.
The results show that the time and the type of atmosphere influence the siderite decomposition process. Regardless of these factors, the process occurs in the temperature range of 300–400 °C. However, the longer the heating time, the faster the siderite decomposition process begins, and its products are iron oxides (hematite and magnetite).
Author Contributions
Conceptualization, M.K.-G.; methodology, M.K.-G.; software, M.K.-G.; formal analysis, M.K.-G., Z.A. and M.W.; investigation, M.K.-G. and M.W.; writing—original draft preparation, M.K.-G.; writing—review and editing, M.K.-G.; visualization, M.K.-G.; supervision, M.K.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data may be made available by the authors upon individual request.
Acknowledgments
I would like to thank Jacek Nowak from the Silesian Technical University (Gliwice, Poland) for providing the material for research and Joanna Klimontko from the University of Silesia (Chorzów, Poland) for performing X-ray diffraction measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halama, M.; Swanner, E.; Konhauser, K.; Kappler, A. Evaluation of siderite and magnetite formation in BIFs by pressure–temperature experiments of Fe(III) minerals and microbial biomass. EPSL 2016, 450, 243–253. [Google Scholar] [CrossRef]
- Cornelis, K. Some Precambrian Banded Iron-Formations (BiIFs) from around the World: Their Age, Geologic Setting, Mineralogy, Metamorphism, Geochemistry, and Origin. Am. Min. 2005, 90, 1473–1499. [Google Scholar]
- Cerantola, V.; McCammon, C.; Kupenko, I.; Kantor, I.; Marini, C.; Wilke, M.; Ismailova, L.; Solopova, N.; Chumakov, A.; Pascarelli, S.; et al. High-pressure spectroscopic study of siderite (FeCO3) with a focus on spin crossover. Am. Min. 2015, 100, 2670–2681. [Google Scholar] [CrossRef]
- Kelektsoglou, K. Carbon capture and storage: A review of mineral storage of CO2 in Greece. Sustainability 2018, 10, 4400. [Google Scholar] [CrossRef]
- Mendoza, M.E.Y.; Santos, A.S.; López, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Fosbol, P.L.; Thomsen, K.; Stenby, E.H. Review and recommended thermodynamic properties of FeCO3. Corros. Eng. Sci. Technol. 2010, 45, 115–135. [Google Scholar] [CrossRef]
- Santoso, R.K.; Rahmawati, S.D.; Gadesa, A.; Wahyuningrum, D. Understanding Passive Layer Formation for Further Corrosion Management in Gas Production Pipes. J. Phys. Conf. Ser. 2017, 877, 012062. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, Y.; Pan, J.; Zhou, X. Reaction Mechanism of Siderite Lump in Coal-Based Direct Reduction. High Temp. Mater. Proc. 2016, 35, 185–194. [Google Scholar] [CrossRef]
- Kholodov, V.N.; Butuzova, G.Y. Siderite Formation and Evolution of Sedimentary Iron Ore Deposition in the Earth’s History. Geol. Ore Depos. 2008, 50, 299–319. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Sun, Y.; Gao, P.; Li, Y. Thermal Decomposition of Siderite Ore in Different Flowing Atmospheres: Phase Transformation and Magnetism. Miner. Process. Extr. Metall. Rev. 2023, 44, 201–208. [Google Scholar] [CrossRef]
- Fisher, Q.; Raiswell, R.; Marshall, J. Siderite concretions from nonmarine shales (Westphalian A) of the Pennines, England: Controls on their growth and composition. J. Sediment. Res. 1998, 68, 1034–1045. [Google Scholar] [CrossRef]
- Gotor, F.J.; Macias, M.; Ortega, A.; Criado, J.M. Comparative study of the kinetics of the thermal decomposition of synthetic and natural siderite samples. Phys. Chem. Miner. 2000, 27, 495–503. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, Y.; Liu, G.; Chen, W. Kinetics of the thermal decomposition of Wangjiatan siderite. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2011, 26, 523–526. [Google Scholar] [CrossRef]
- De Grave, E.; Eeckhout, S.G.; McCammon, C.A. Selected applications of 57Fe Mossbauer spectroscopy to mineral studies. Hyperfine Interact. 1999, 122, 21–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).