Electrochemical Multisensor System for Monitoring the Hydrogen Peroxide Direct Synthesis in Microreactors †
Abstract
:1. Introduction
2. Materials and Methods
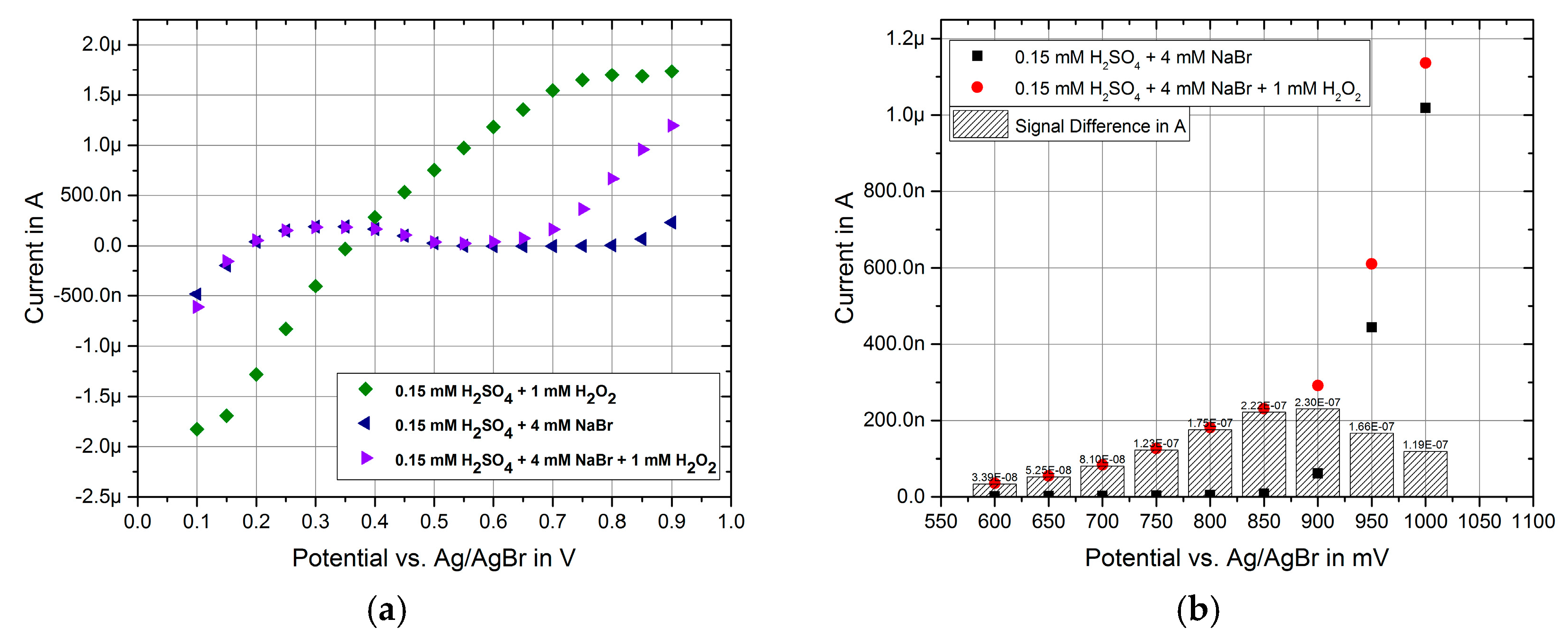
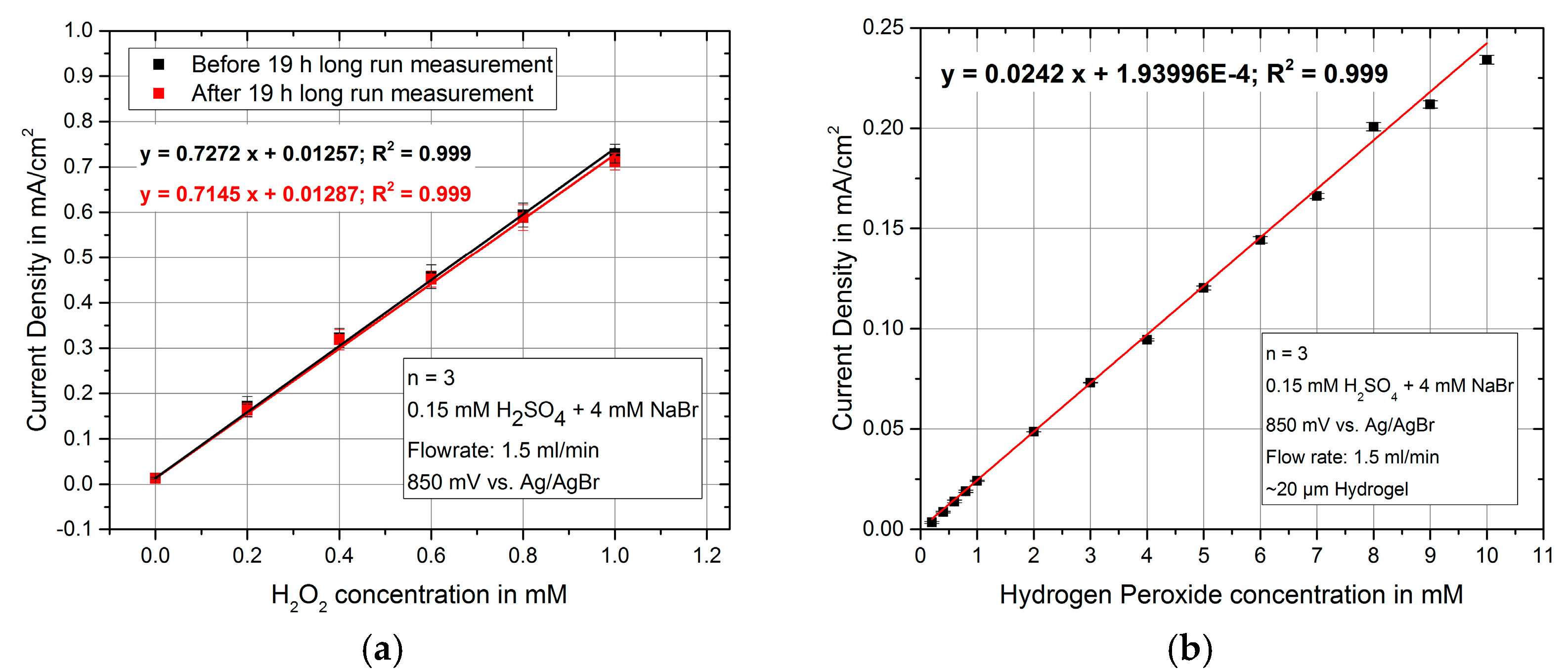
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Dittmeyer, R.; Grunwaldt, J.-D.; Pashkova, A. A review of catalyst performance and novel reaction engineering concepts in direct synthesis of hydrogen peroxide. Catal. Today 2015, 248, 149–159. [Google Scholar] [CrossRef]
- Weltin, A.; Enderle, B.; Kieninger, J.; Urban, G.A. Multiparametric, Flexible Microsensor Platform for Metabolic Monitoring In Vivo. IEEE Sens. J. 2014, 14, 3345–3351. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, S.; Weltin, A.; Flamm, H.; Kieninger, J.; Deschner, B.J.; Kraut, M.; Dittmeyer, R.; Urban, G.A. Electrochemical Multisensor System for Monitoring the Hydrogen Peroxide Direct Synthesis in Microreactors. Proceedings 2017, 1, 630. https://doi.org/10.3390/proceedings1040630
Urban S, Weltin A, Flamm H, Kieninger J, Deschner BJ, Kraut M, Dittmeyer R, Urban GA. Electrochemical Multisensor System for Monitoring the Hydrogen Peroxide Direct Synthesis in Microreactors. Proceedings. 2017; 1(4):630. https://doi.org/10.3390/proceedings1040630
Chicago/Turabian StyleUrban, Sebastian, Andreas Weltin, Hubert Flamm, Jochen Kieninger, Benedikt J. Deschner, Manfred Kraut, Roland Dittmeyer, and Gerald A. Urban. 2017. "Electrochemical Multisensor System for Monitoring the Hydrogen Peroxide Direct Synthesis in Microreactors" Proceedings 1, no. 4: 630. https://doi.org/10.3390/proceedings1040630
APA StyleUrban, S., Weltin, A., Flamm, H., Kieninger, J., Deschner, B. J., Kraut, M., Dittmeyer, R., & Urban, G. A. (2017). Electrochemical Multisensor System for Monitoring the Hydrogen Peroxide Direct Synthesis in Microreactors. Proceedings, 1(4), 630. https://doi.org/10.3390/proceedings1040630






