Real-Time Monitoring of Stem Cells by Diamond-Based Impedance Sensors †
Abstract
:1. Introduction
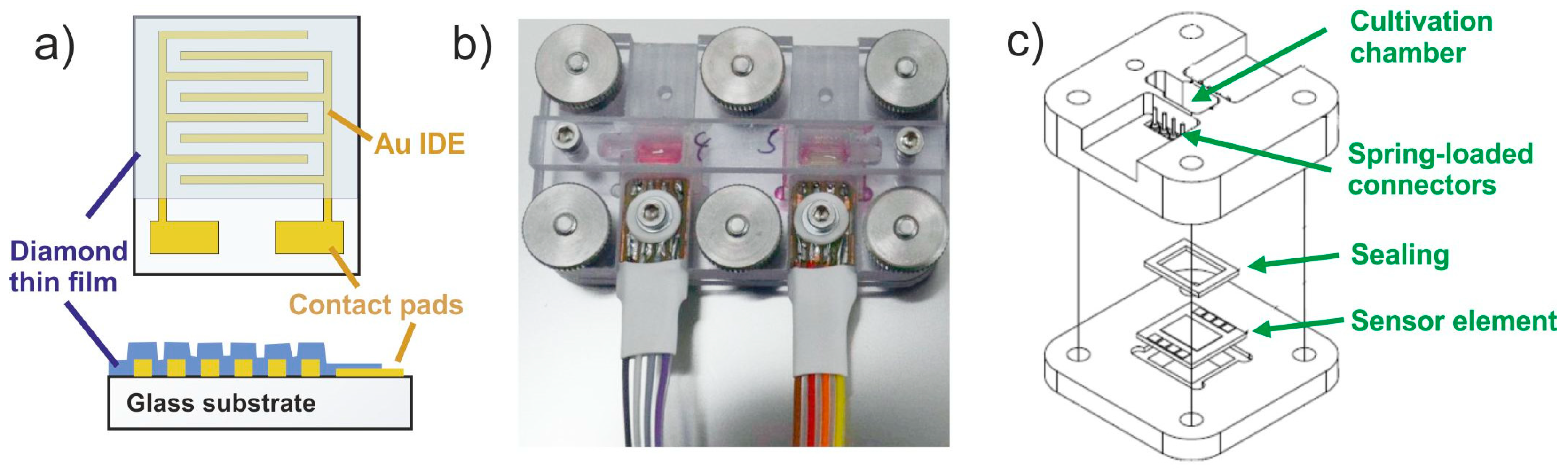
2. Materials and Methods
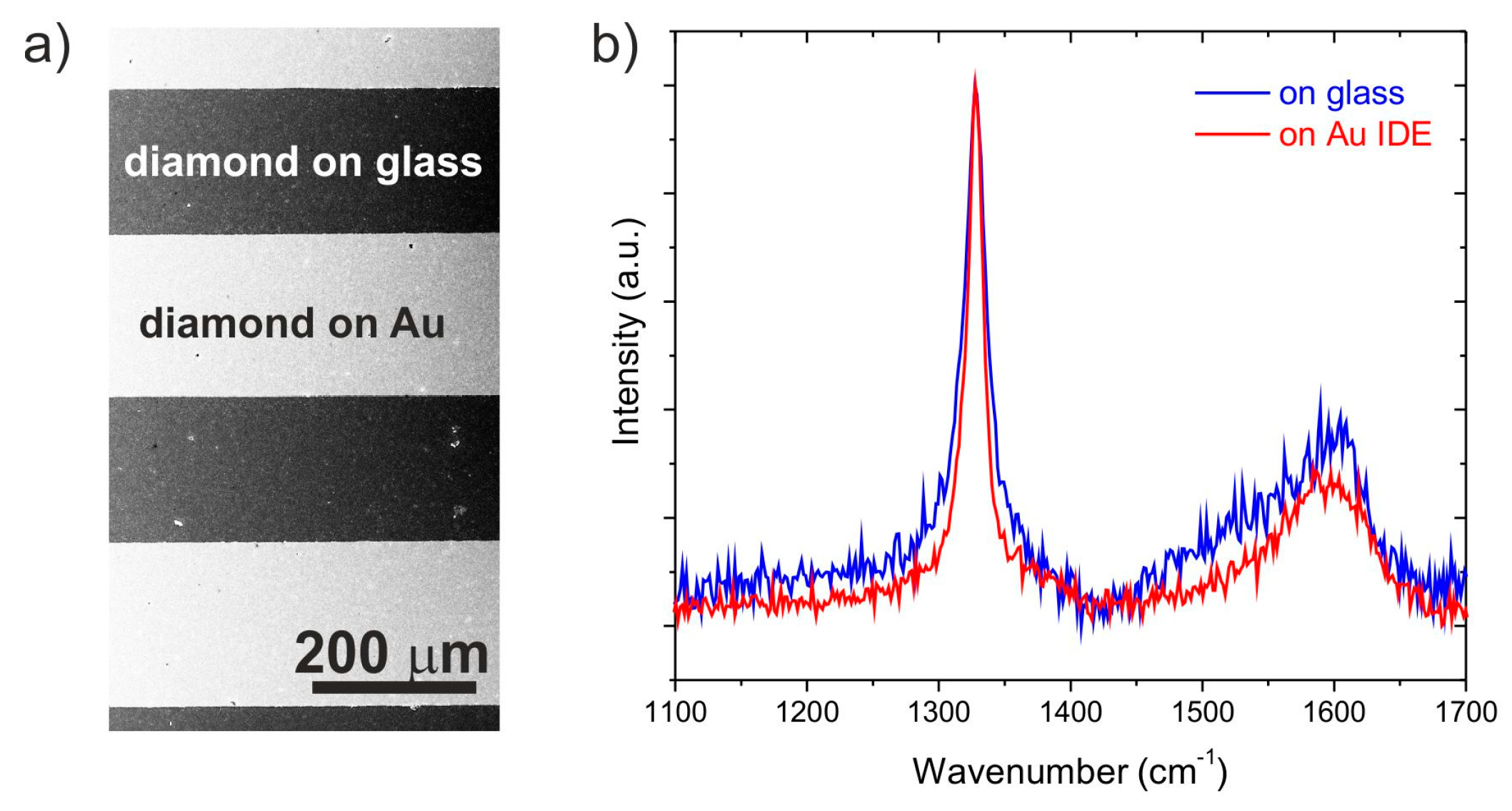
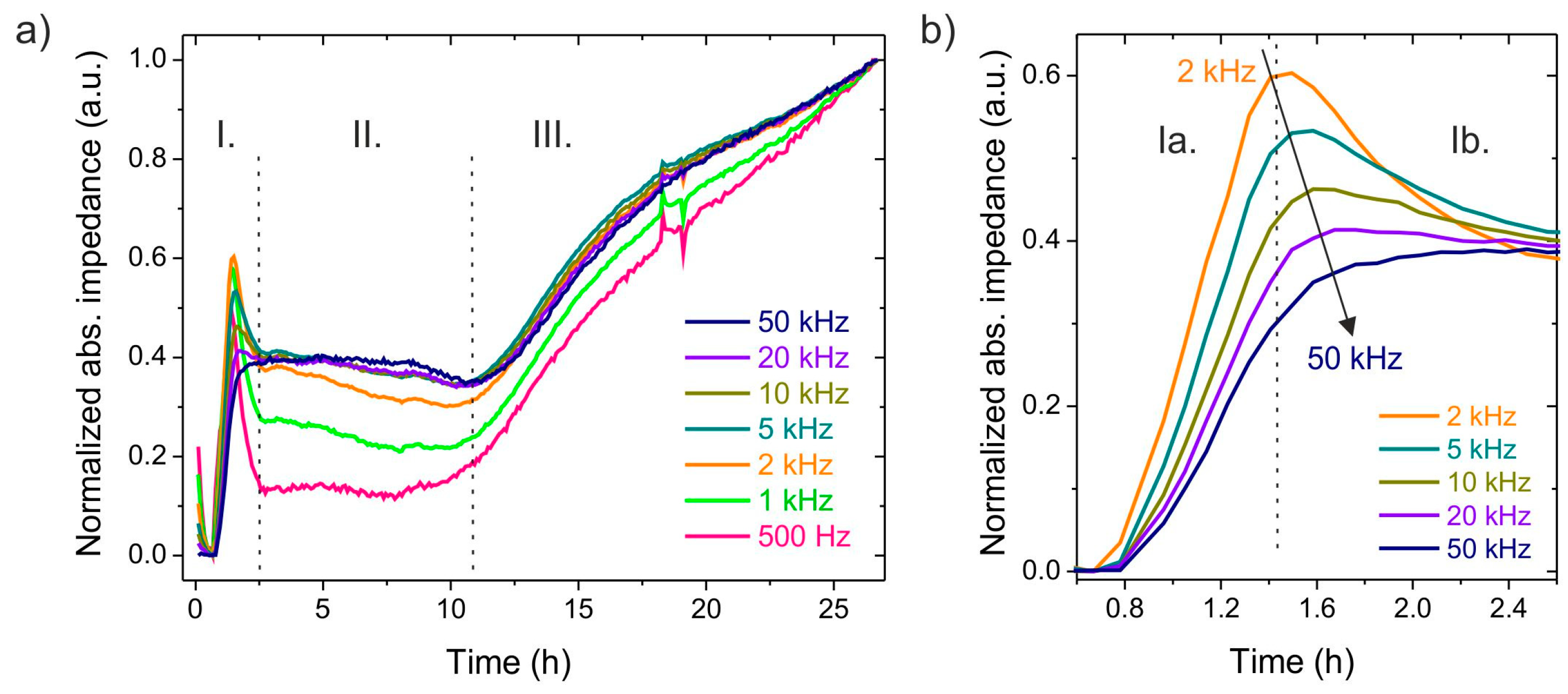
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fang, Y. Label-Free Biosensors for Cell Biology. Int. J. Electrochem. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Guan, N.; Deng, J.; Li, T.; Xu, X.; Irelan, J.T.; Wang, M.-W. Label-free monitoring of T cell activation by the impedance-based xCELLigence system. Mol. Biosyst. 2013, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Verdanova, M.; Rezek, B.; Broz, A.; Ukraintsev, E.; Babchenko, O.; Artemenko, A.; Izak, T.; Kromka, A.; Kalbac, M.; Hubalek Kalbacova, M. Nanocarbon Allotropes-Graphene and Nanocrystalline Diamond-Promote Cell Proliferation. Small 2016, 12, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Izak, T.; Novotna, K.; Kopova, I.; Bacakova, L.; Rezek, B.; Kromka, A. H-terminated diamond as optically transparent impedance sensor for real-time monitoring of cell growth: H-terminated diamond as optically transparent impedance sensor. Phys. Status Solidi B 2013, 250, 2741–2746. [Google Scholar] [CrossRef]
- Davydova, M.; Stuchlik, M.; Rezek, B.; Larsson, K.; Kromka, A. Sensing of phosgene by a porous-like nanocrystalline diamond layer with buried metallic electrodes. Sens. Actuators B Chem. 2013, 188, 675–680. [Google Scholar] [CrossRef]
- Stehlik, S.; Izak, T.; Kromka, A.; Dolenský, B.; Havlík, M.; Rezek, B. Sensitivity of Diamond-Capped Impedance Transducer to Tröger’s Base Derivative. ACS Appl. Mater. Interfaces 2012, 4, 3860–3865. [Google Scholar] [CrossRef] [PubMed]
- Potocký, Š.; Čada, M.; Babchenko, O.; Ižák, T.; Davydova, M.; Kromka, A. Perspectives of linear antenna microwave system for growth of various carbon nano-forms and its plasma study: Perspectives of linear antenna microwave system. Phys. Status Solidi B 2013, 250, 2723–2726. [Google Scholar] [CrossRef]
- Prawer, S.; Nemanich, R.J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2004, 362, 2537–2565. [Google Scholar] [CrossRef] [PubMed]
- Applied Biophysics. Available online: http://www.biophysics.com/ecis-theory.php (accessed on 20 June 2017).
- Ižák, T.; Szabó, O.; Bačáková, L.; Kromka, A. Diamond Functional Layers for Cell-based Impedance Spectroscopy. Procedia Eng. 2016, 168, 614–617. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procházka, V.; Matějka, R.; Ižák, T.; Szabó, O.; Štěpanovská, J.; Bačáková, L.; Kromka, A. Real-Time Monitoring of Stem Cells by Diamond-Based Impedance Sensors. Proceedings 2017, 1, 515. https://doi.org/10.3390/proceedings1040515
Procházka V, Matějka R, Ižák T, Szabó O, Štěpanovská J, Bačáková L, Kromka A. Real-Time Monitoring of Stem Cells by Diamond-Based Impedance Sensors. Proceedings. 2017; 1(4):515. https://doi.org/10.3390/proceedings1040515
Chicago/Turabian StyleProcházka, Václav, Roman Matějka, Tibor Ižák, Ondrej Szabó, Jana Štěpanovská, Lucie Bačáková, and Alexander Kromka. 2017. "Real-Time Monitoring of Stem Cells by Diamond-Based Impedance Sensors" Proceedings 1, no. 4: 515. https://doi.org/10.3390/proceedings1040515
APA StyleProcházka, V., Matějka, R., Ižák, T., Szabó, O., Štěpanovská, J., Bačáková, L., & Kromka, A. (2017). Real-Time Monitoring of Stem Cells by Diamond-Based Impedance Sensors. Proceedings, 1(4), 515. https://doi.org/10.3390/proceedings1040515







