1. Introduction
The aim of this work is to develop an integrated flexible large-scale sensing platform using low cost fabrication techniques (screen printing and dispensing) for analyzing multiple ions in fluids. This low cost flexible platform for monitoring multiple ions sensing could be beneficial to measure and control certain human physiological conditions, like salt intake, physical stress and disease diagnosis [
1,
2].
We introduced an earlier prototype of the miniaturized ion sensors with an integrated hydrogel polyhydroxyethylmethacrylate (pHEMA) based RE for potassium and sodium sensing [
3]. In these sensors, the hydrogel pHEMA was both used as the internal electrolyte for the reference electrode and for the ion-selective electrodes. Integrated in a patch we demonstrated real-time monitoring of chloride concentration in sweat [
4]. The hydrogel pHEMA has drawbacks however: it requires UV curing and conditioning in fluid for the KCl loading before usage [
4]. Furthermore, the hydrogel is in direct contact with the sample and therefore chloride ions leach out slowly from the internal electrolyte layer leading to drift of the reference electrode. To slow down the depletion of the chloride concentration in the reference electrode, an O-ring was used to form a reservoir and to increase the pHEMA layer thickness.
In this work, a new design is introduced for the flexible reference and ion-selective electrodes, in which the reservoirs for the internal electrolytes of these electrodes were created by laminating a thermoplastic polyurethane (TPU) layer with wells. This enables large scale fabrication of the sensor array. Furthermore, to minimize the depletion of the chloride concentration in the thin internal electrolyte layer of the reference electrode further, the hydrogel pHEMA was replaced by plasticized PVC premixed with KCl. In the ISEs, the hydrogel pHEMA internal electrolyte was replaced by the cellulose gel preloaded with 0.1 M KCl, preventing conditioning the electrodes in fluid beforehand. The drift of the reference electrode and sensitivity of potassium-selective sensor both fabricated on the same foil substrate were characterized.
2. Materials and Methods Section
An array of AgCl electrodes was screen printed on the polyethylene terephthalate (PET) foil using DuPont 5876 AgCl conducting paste. After the printing, the AgCl layer were dried at 110 °C for three minutes and a next AgCl layer was printed to get a thickness of 50 mm.
To protect the conducting paths and to form the reservoirs for the internal electrolytes, the foil was subsequently laminated with a 600 μm thick TPU layer, which was patterned by laser cutting leaving wells with a diameter of 3 mm above the electrode area open.
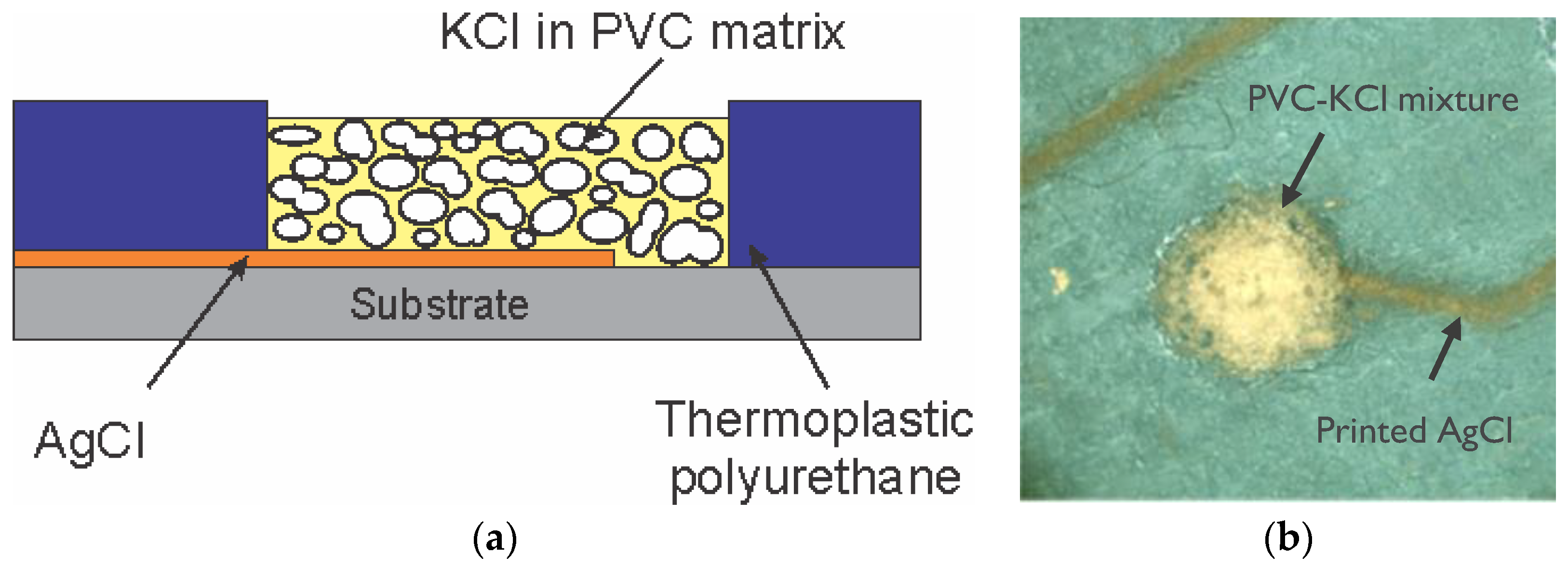
The solid-contact reference electrode was made by drop casting a mixture of PVC, dioctyl sebacate (DOS) plasticizer (PVC:DOS weight ratio of 1:2), KCl (41 wt %) and cyclohexanone inside the RE reservoir as illustrated in
Figure 1a. A potassium-selective electrode was fabricated with cellulose hydrogel as internal electrolyte, which contained 2 wt % hydroxyethyl cellulose (HEC) and 21 wt % triethylene glycol dissolved in 0.1 M KCl. After casting the hydrogel layer, the ion-selective membrane solution, which was prepared according to [
3] was drop casted inside the reservoir on top of the hydrogel layer. The ion-selective membrane solution casting was repeated three times till an optimal thickness of the membrane was achieved. After drying overnight, the reference electrode and ion selective electrodes were ready to test.
The stability of the reference electrode was studied in a 0.1 M KCl solution versus a 3 M KCl commercial reference electrode (CRISON 5044). Calibration of the ion-selective electrodes was performed with respect to the reference electrode made on the same foil substrate in a series of test solutions containing 0.006–0.1 M KCl and other salts (CaCl2 and NaCl) as a supporting electrolyte. All chemicals used were of analytical grade.
3. Results and Discussion
3.1. Reference Electrode
Figure 1b shows a top view photo of a solid-contact reference electrode with plasticized PVC premixed with KCl casted inside the reference electrode reservoir. The KCl crystals were uniformly distributed in the PVC mixture forming the solid contact layer (
Figure 1a).
Upon immersing the RE in fluid, the solution can quickly penetrate in the solid contact layer through the thin PVC layer coverings the KCl crystals which then partially dissolve. As KCl dissociates, it provides a stable chloride concentration above the AgCl electrode in the RE, that resulted in stable RE potential after a few seconds after immersion.
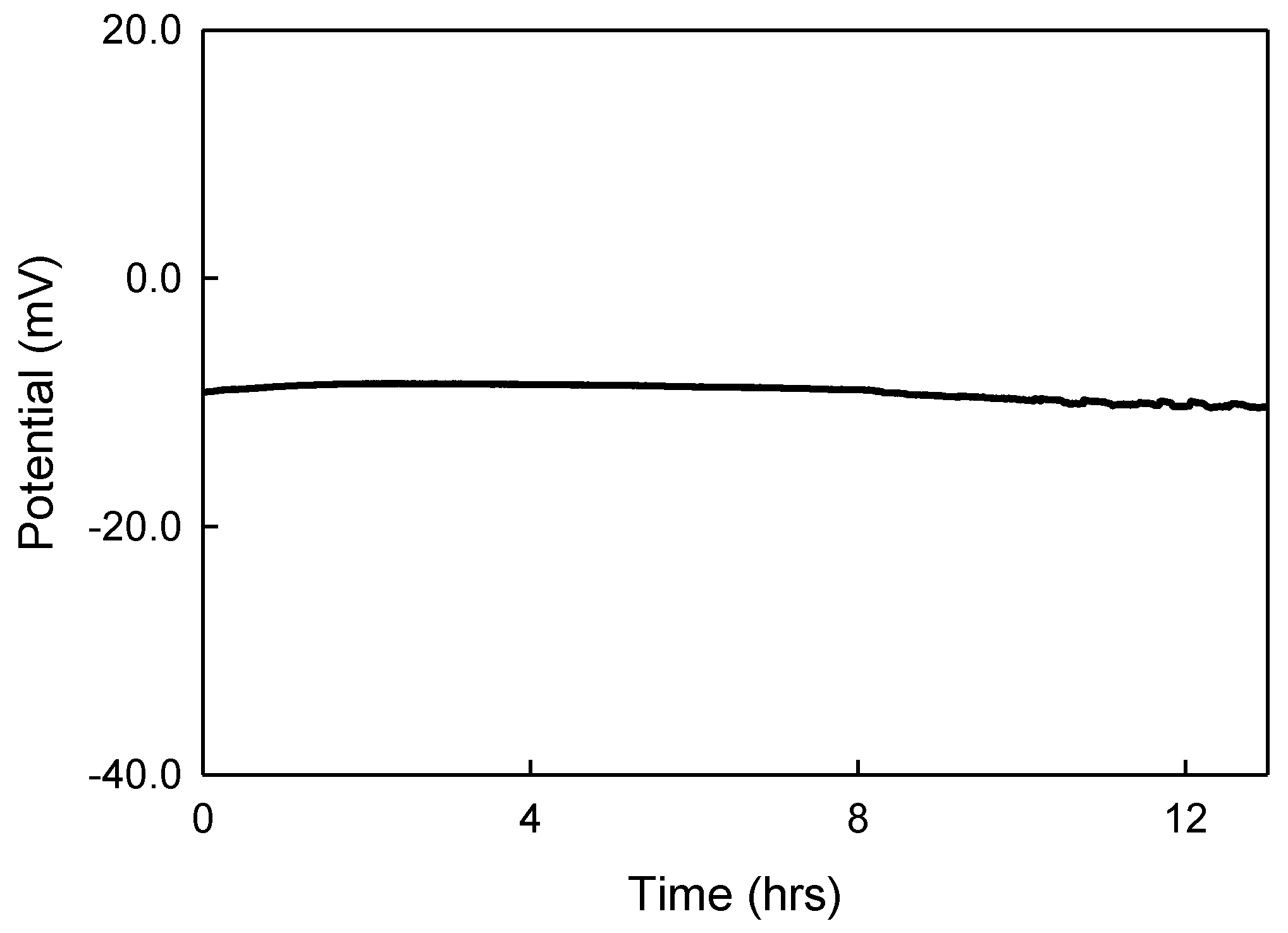
Figure 2 shows the potential of the solid-contact reference electrode versus the Crison Ag/AgCl reference electrode in a 13 h. stability measurement. Chloride ions still diffuse out of the RE, but this loss was immediately replenished by ions dissolving from the an excess amount of KCl crystals in the RE. In the first 13 h, the solid-contact reference electrode showed a stable potential of −9.1 ± 0.6 mV versus the commercial 3 M KCl reference electrode suggesting that the chloride concentration in the vicinity of the AgCl electrode in the reference electrode was indeed close to the saturation level. This stability time was approximately 40 times longer than that of the pHEMA based RE with the reservoir created by the O-ring. After 13 h, the potential of the RE started to rise indicating that the chloride concentration in the solid-contact reference electrode was depleted. The results of the stability measurement in
Figure 2 suggest that in the applications, when the solid-contact reference electrode is not continuously exposed to the solution, the stability time of the RE can be extended further.
3.2. Ion-Selective Electrode
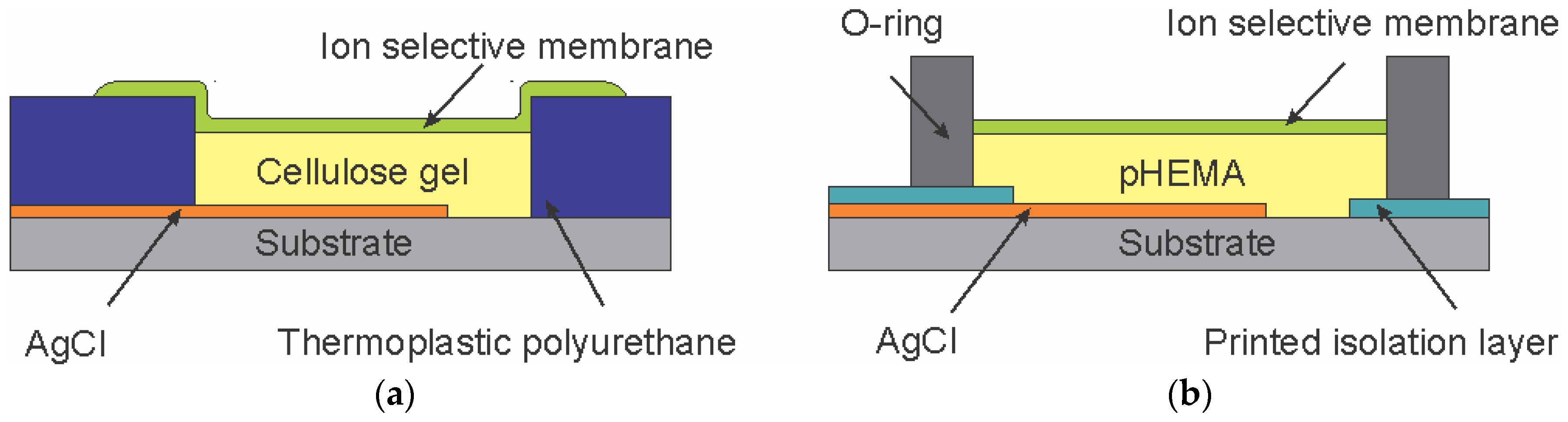
Figure 3 shows an illustration of the cross section of a flexible potassium-selective electrode with an internal electrolyte layer of cellulose gel drop casted in a reservoir formed by the laminated TPU layer. In this sensor design, the cellulose gel preloaded with 0.1 M KCl was used to replace the pHEMA hydrogel (shown in
Figure 3b for comparison), thus the potassium-selective membrane could be drop casted immediately after applying the cellulose hydrogel layer.
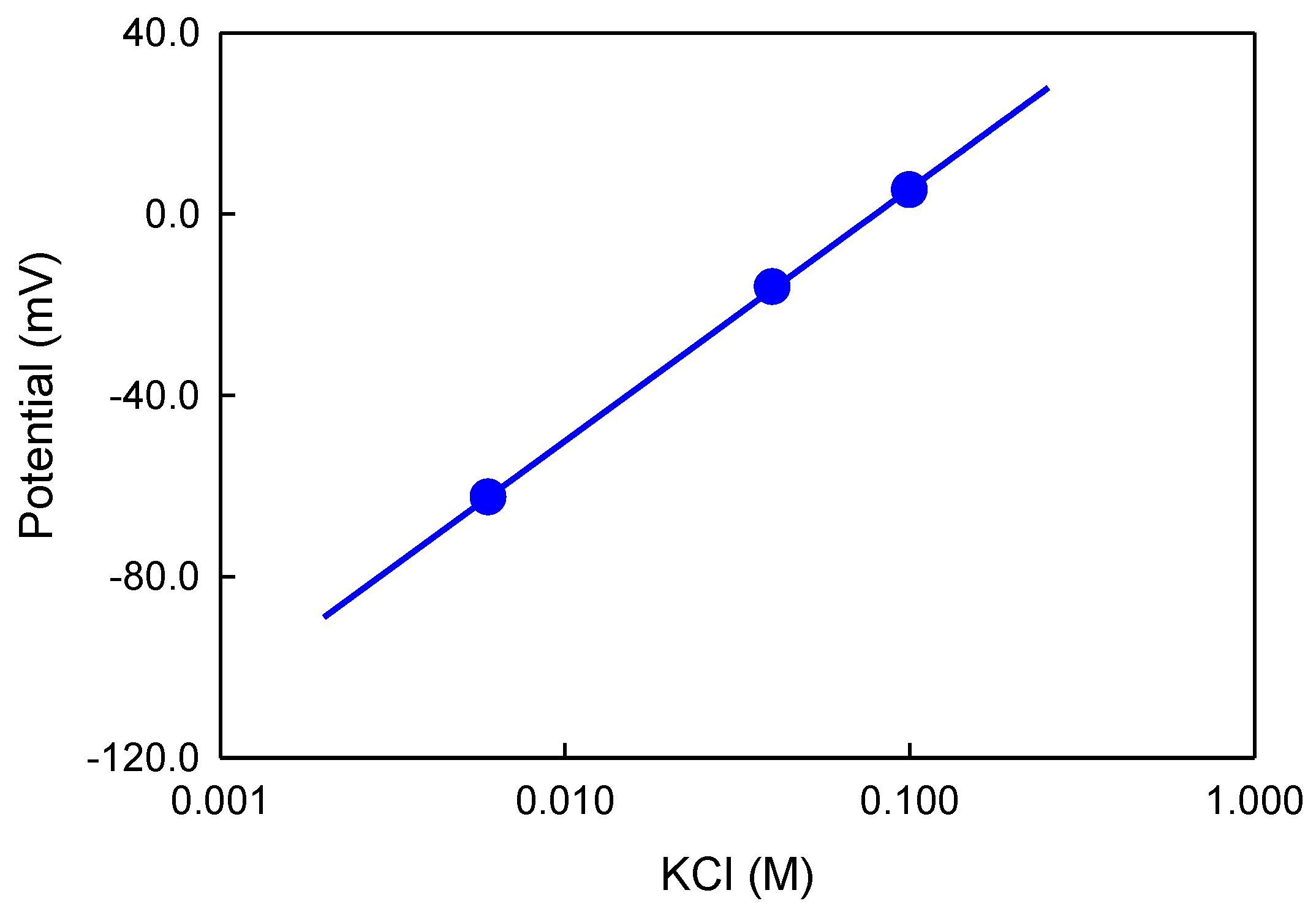
The sensitivity of the potassium sensor with the cellulose gel was determined by measuring its electrode potential in solutions with varied KCl concentration in presence of NaCl (6–300 mM) and CaCl
2 (10–20 mM) as supporting electrolyte versus the solid-contact reference electrode. By plotting the steady-state electrode potential as a function of the KCl concentration (see
Figure 4) and using a linear fit, the potassium sensitivity was calculated. In a concentration range of 6–100 mM KCl, which is close to the potassium concentration in bodily fluids, the sensor showed a sensitivity of 56 mV per decade in presence of CaCl
2 and NaCl. Extension of this sensing platform to other ions will follow.
4. Conclusions
A flexible sensor consisting of a potassium-selective electrode having a cellulose gel internal electrolyte and stable solid-contact reference electrode was fabricated using screen printing and drop casting techniques. The reference electrode, which did not require stabilization time, showed a stable potential of −9.1 ± 0.6 mV in 13 h continuous drift test. The potassium-selective electrode, which was made on the same foil showed the sensitivity of 56 mV/decade versus the solid-contact reference electrode. The sensitive range of the potassium sensor is well suited for bodily fluids analysis.