Influence of Fertigation Regimes on Nitrogen Concentration in Apple (Malus × domestica Borkh.) Leaves at Different Age Stages
Abstract
1. Introduction
2. Materials and Methods
- Treatment 1 (H1): Hoagland solution, all macronutrients and micronutrients;
- Treatment 2 (H2): Hoagland solution N excluded;
- Treatment 3 (H3): Hoagland solution Fe excluded;
- Treatment 4 (H4): Hoagland solution Mg excluded.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| Ca | Calcium |
| Mg | Magnesium |
| Fe | Iron |
| HS | Hoagland solution |
| H1 | Treatment 1 |
| H2 | Treatment 2 |
| H3 | Treatment 3 |
| H4 | Treatment 4 |
References
- Cornille, A.; Gladieux, P.; Smulders, M.J.; Roldán-Ruiz, I.; Laurens, F.; Le Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties. PLoS. Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/items/5c272dc7-e1b8-486a-b323-6babb174eee0 (accessed on 2 September 2025).
- Ahad, S.; Mir, M.M.; Ashraf, S.; Mumtaz, S.; Hamid, M. Nutrient Management in High Density Apple Orchards—A Review. Curr. J. Appl. Sci. Technol. 2018, 29, 1–16. [Google Scholar] [CrossRef]
- Rengel, Z.; Cakmak, I.; White, P.J. Marschner’s Mineral Nutrition of Plants; Academic Press: London, UK, 2023. [Google Scholar]
- Ličina, V.; Krogstad, T.; Simić, A.; Fotirić Akšić, M.; Meland, M. Nutrition and Fertilizer Application to Apple Trees—A Review; NIBIO report; Norwegian Institute of Bioeconomy Research: Ås, Norway, 2021; Volume 7, p. 79. Available online: https://kudos.dfo.no/documents/49618/files/32058.pdf (accessed on 2 October 2025).
- Tripathy, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Kumar Srivastava, A.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Effect of Nitrogen Fertilization of Apple Orchard on Soil Mineral Nitrogen Content, Yielding of the Apple Trees and Nutritional Status of Leaves and Fruits. Agriculture 2022, 12, 2169. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Dominguez, L.I.; Robinson, T.L. Benefits of Irrigation or Fertigation on Early Growth and Yield of a High-density Apple Planting in a Humid Climate. HortTechnology 2024, 34, 747–760. [Google Scholar] [CrossRef]
- Lešić, R.; Borošić, J.; Butorac, I.; Herak Ćustić, M.; Poljak, M.; Romić, D. Povrćarstvo, 3rd revised ed.; Zrinski: Čakovec, Croatia, 2016; ISBN 9789531551335. [Google Scholar]
- Sakuraba, Y. Molecular basis of nitrogen starvation-induced leaf senescence. Front. Plant Sci. 2022, 13, 1013304. [Google Scholar] [CrossRef] [PubMed]
- Javornik, T.; Carović-Stanko, K.; Gunjača, J.; Vidak, M.; Lazarević, B. Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging. Plants 2023, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Herak Ćustić, M.; Petek, M.; Palčić, I.; Herak-Kramberger, C.M. Ishrana Bilja u Hortikulturi, Krajobrazu i Kvaliteti Hrane; Školska knjiga: Zagreb, Croatia, 2025; ISBN 978-953-0-31150-3. [Google Scholar]
- Vukadinović, V.; Vukadinović, V. Ishrana Bilja; Poljoprivredni fakultet u Osijeku: Osijek, Hrvatska, 2011; Volume 3. [Google Scholar]
- Kabange, N.R.; Lee, S.M.; Shin, D.; Lee, J.Y.; Kwon, Y.; Kang, J.W.; Cha, J.K.; Park, H.; Alibu, S.; Lee, J.H. Multiple Facets of Nitrogen: From Atmospheric Gas to Indispensable Agricultural Input. Life 2022, 12, 1272. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional Disorders of Plants: Development, Visual and Analytical Diagnosis; Gustav Fischer: Stuttgart, Germany, 1992. [Google Scholar]
- Cheng, L.; Fuchigami, L.H. Growth of young apple trees in relation to reserve nitrogen and carbohydrates. Tree Physiol. 2002, 22, 1297–1303. [Google Scholar] [CrossRef]
- Zheng Tan, B.; Close, D.C.; Quin, P.R.; Swarts, N.D. Nitrogen use efficiency, allocation, and remobilization in apple trees: Uptake is optimized with pre-harvest N supply. Front. Plant Sci. 2021, 12, 657070. [Google Scholar] [CrossRef]
- Hanson, E.; Tissue Analysis for Monitoring Fruit Nutrition. Michigan State University. MSU Extension. 2014. Available online: https://www.canr.msu.edu/news/tissue_analysis_for_monitoring_fruit_nutrition?utm_source (accessed on 3 September 2025).
- Čoga, L.; Slunjski, S. Dijagnostika tla u Ishrani Bilja: Priručnik za Uzorkovanje i Analitiku tla; Sveučilište u Zagrebu Agronomski fakultet: Zagreb, Croatia, 2018; Available online: https://urn.nsk.hr/urn:nbn:hr:204:925835 (accessed on 4 September 2025).
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The significance of microbial transformation of nitrogen compounds in the light of integrated crop management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Postma, J.A.; Hecht, V.L.; Hikosaka, K.; Nord, E.A.; Pons, T.L.; Poorter, H. Dividing the pie: A quantitative review on plant density responses. Plant Cell Environ. 2021, 44, 1072–1094. [Google Scholar] [CrossRef]
- Cai, S.; Zheng, B.; Zhao, Z.; Zheng, Z.; Yang, N.; Zhai, B. Precision Nitrogen Fertilizer and Irrigation Management for Apple Cultivation Based on a Multilevel Comprehensive Evaluation Method of Yield, Quality, and Profit Indices. Water 2023, 15, 468. [Google Scholar] [CrossRef]
- Jang, S.; Han, J.; Cho, J.; Jung, J.; Lee, S.; Lee, D.; Kim, J. Estimation of Apple Leaf Nitrogen Concentration Using Hyperspectral Imaging-Based Wavelength Selection and Machine Learning. Horticulturae 2024, 10, 35. [Google Scholar] [CrossRef]
- Nielsen, G.H.; Neilsen, D.; Herbert, L. Nitrogen Fertigation Concentration and Timing of Application Affect Nitrogen Nutrition, Yield, Firmness, and Color of Apples Grown at High Density. HortScience 2009, 44, 1425–1431. [Google Scholar] [CrossRef]
- De Angelis, V.; Sánchez, E.; Tognetti, J. Timing of Nitrogen Fertilization Influences Color and Anthocyanin Content of Apple (Malus domestica Borkh. cv ‘Royal Gala’) Fruits. Int. J. Fruit Sci. 2011, 11, 364–375. [Google Scholar] [CrossRef]
- Cheng, L. When and How Much Nitrogen Should Be Applied in Apple Orchards? N. Y. Fruit Q. 2010, 18, 4. Available online: https://nyshs.org/wp-content/uploads/2016/10/5.When-and-How-Much-Nitrogen-Should-Be-Applied-in-Apple-Orchards.pdf?utm_source (accessed on 10 October 2025).
- Dong, S.; Scagel, C.F.; Cheng, L.; Fuchigami, L.H.; Rygiewicz, P.T. Soil temperature and plant growth stage influence nitrogen uptake and amino acid concentration of apple during early spring growth. Tree Physiol. 2021, 21, 541–547. [Google Scholar] [CrossRef]
- Chang, C.; Li, C.; Li, C.; Kang, X.; Zou, Y.; Ma, F. Differences in the efficiency of potassium (K) uptake and use in five apple rootstock genotypes. J. Integr. Agric. 2014, 13, 1934–1942. [Google Scholar] [CrossRef]
- Biasuz, E.C.; Kalcsits, L. Rootstock effects on leaf function and isotope composition in apple occurred on both scion grafted and ungrafted rootstocks under hydroponic conditions. Front. Plant Sci. 2023, 14, 1274195. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 2nd ed.; California Agricultural Experiment Station: Berkeley, CA, USA, 1950. [Google Scholar]
- HRN EN ISO/IEC 17025:2017; Opći Zahtjevi za Osposobljenost Ispitnih i Umjernih Laboratorija (ISO/IEC 17025:2017; EN ISO/IEC 17025:2017). Hrvatski Normativni Document; Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2017. Available online: https://repozitorij.hzn.hr/norm/HRN+EN+ISO%2FIEC+17025%3A2017 (accessed on 11 October 2025).
- AOAC International. Official Methods of Analysis of AOAC International, 22nd ed.; Oxford University Press: Oxford, UK, 2023; ISBN 978-0-19-764908-4/978-0-19-764909-1/978-0-19-764910-7. [Google Scholar]
- Neubert, P.; Wrazidlo, W.; Vielemeyer, H.P.; Hundt, I.; Gollmick, F.; Bergmann, W. Tables of Plant Analysis; Institute of Plant Nutrition: Peachtree Corners, GA, USA, 1970. [Google Scholar]
- Crassweller, R. Orchard Nutrition: An Overview. 2023. Available online: https://extension.psu.edu/orchard-nutrition-an-overview (accessed on 25 August 2025).
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; 849p. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Chaganzhana; Mabuchi, A.; Iba, M.; Yanagisawa, S. Enhanced NRT1.1/NPF6.3 expression in shoots improves growth under nitrogen deficiency stress in Arabidopsis. Commun. Biol. 2021, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: London, UK, 2012; pp. 191–200. [Google Scholar]
- Hermans, C.; Vebruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Aguirre, P.B.; Al-Hinai, Y.K.; Roper, T.R.; Krueger, A.R. Apple Tree Rootstock and Fertilizer Application Timing Affect Nitrogen Uptake. HortScience 2001, 36, 1202–1205. [Google Scholar] [CrossRef]
- Neto, C.; Carranca, C.; Clemente, J.; De Varennes, A. Nitrogen distribution, remobilization and re-cycling in young orchard of non-bearing ‘Rocha’ pear trees. Sci. Hortic. 2008, 118, 299–307. [Google Scholar] [CrossRef]
- Neilsen, D.; Millard, P.; Neilsen, G.H.; Hogue, E.J. Sources of N for leaf growth in a high density apple (Malus domestica) orchard irrigated with an ammonium nitrate solution. Tree Physiol. 1997, 17, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Millard, P. Ecophysiology of the internal cycling of nitrogen for tree growth. J. Plant Nutr. Soil Sci. 1996, 159, 1–10. [Google Scholar] [CrossRef]
- Millard, P.; Grelet, G. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef]
- Faust, M. Physiology of Temperate Zone Fruit Trees; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Tagliavini, M.; Scandellari, F. Methodologies and concepts in the study of nutrient uptake requirements and partitioning in fruit trees. In ISHS Acta Horticulturae 984, Proceedings of the VII International Symposium on Mineral Nutrition of Fruit Crops, Chanthaburi, Thailand, 19–25 May 2012; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2012. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, an Introduction to Nutrient Management, 8th ed.; Pearson: Boston, MA, USA, 2014; Available online: https://www.researchgate.net/profile/Praveen-Kumar-521/publication/366175716_Soil_Fertility_and_Fertilizers_by_John_L_Havlin_z-liborg/links/63948fbde42faa7e75af15db/Soil-Fertility-and-Fertilizers-by-John-L-Havlin-z-liborg.pdf (accessed on 22 August 2025).
- Neilsen, G.H.; Neilsen, D. Nutritional requirements of apple. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.A., Eds.; CAB International: Wallingford, UK, 2003; pp. 267–302. Available online: https://www.taylorfrancis.com/books/edit/10.4324/9781351114233/achieving-sustainable-cultivation-apples-kate-evans?refId=cc36e60c-1b62-4c81-8f7b-1a91c16ce900&context=ubx (accessed on 28 September 2025).
- Treder, W.; Klamkowski, K.; Wójcik, K.; Tryngiel-Gać, A.; Sas-Paszt, L.; Mika, A.; Kowalczyk, W. Apple leaf macro-and micronutrient content as affected by soil treatments with fertilizers and microorganisms. Sci. Hortic. 2022, 297, 110975. [Google Scholar] [CrossRef]
- Tagliavini, M.; Marangoni, B. Major nutritional issues in deciduous fruit orchards in Northern Italy. HortTechnology 2002, 12, 26–31. [Google Scholar] [CrossRef]
- Hanson, E.; MSU—Michigan State University; MSU Extension. Apples. Apple Nutrition. Available online: https://www.canr.msu.edu/uploads/files/Applenutrition-EricHanson.pdf (accessed on 20 August 2025).
- Au Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-L.; Wang, Z.-J.; Yin, X.-W.; Sun, Y.-L.; Jang, D.-J.; Hong, S.-K. The impact of nitrogen deposition on nitrogen metabolism in ryegrass lawn with different soil nutrient levels. Sci. Rep. 2025, 15, 16755. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Content of minerals in soil, apple tree leaves and fruits depending on nitrogen fertilization. J. Elem. 2017, 22, 67–77. [Google Scholar] [CrossRef]

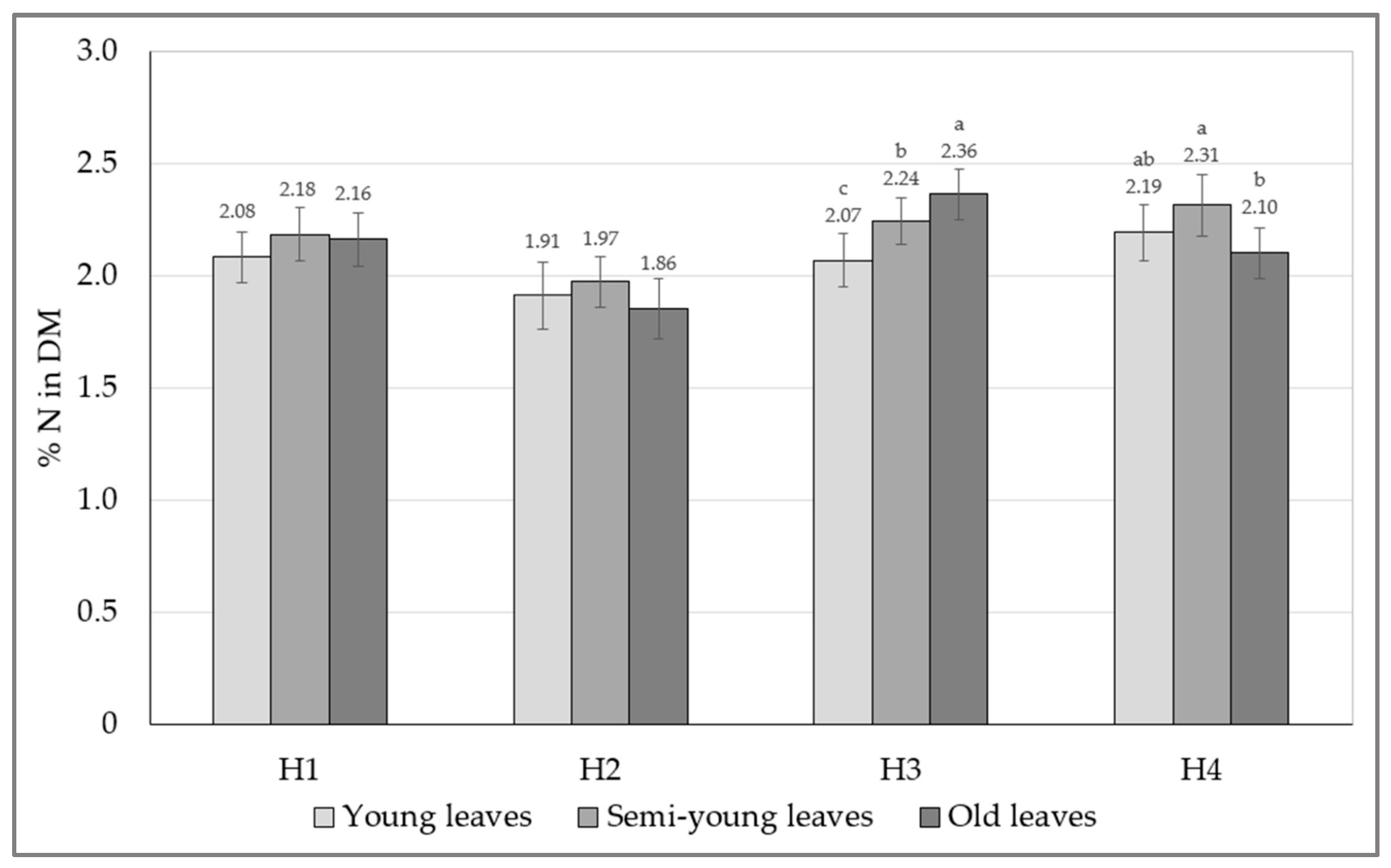
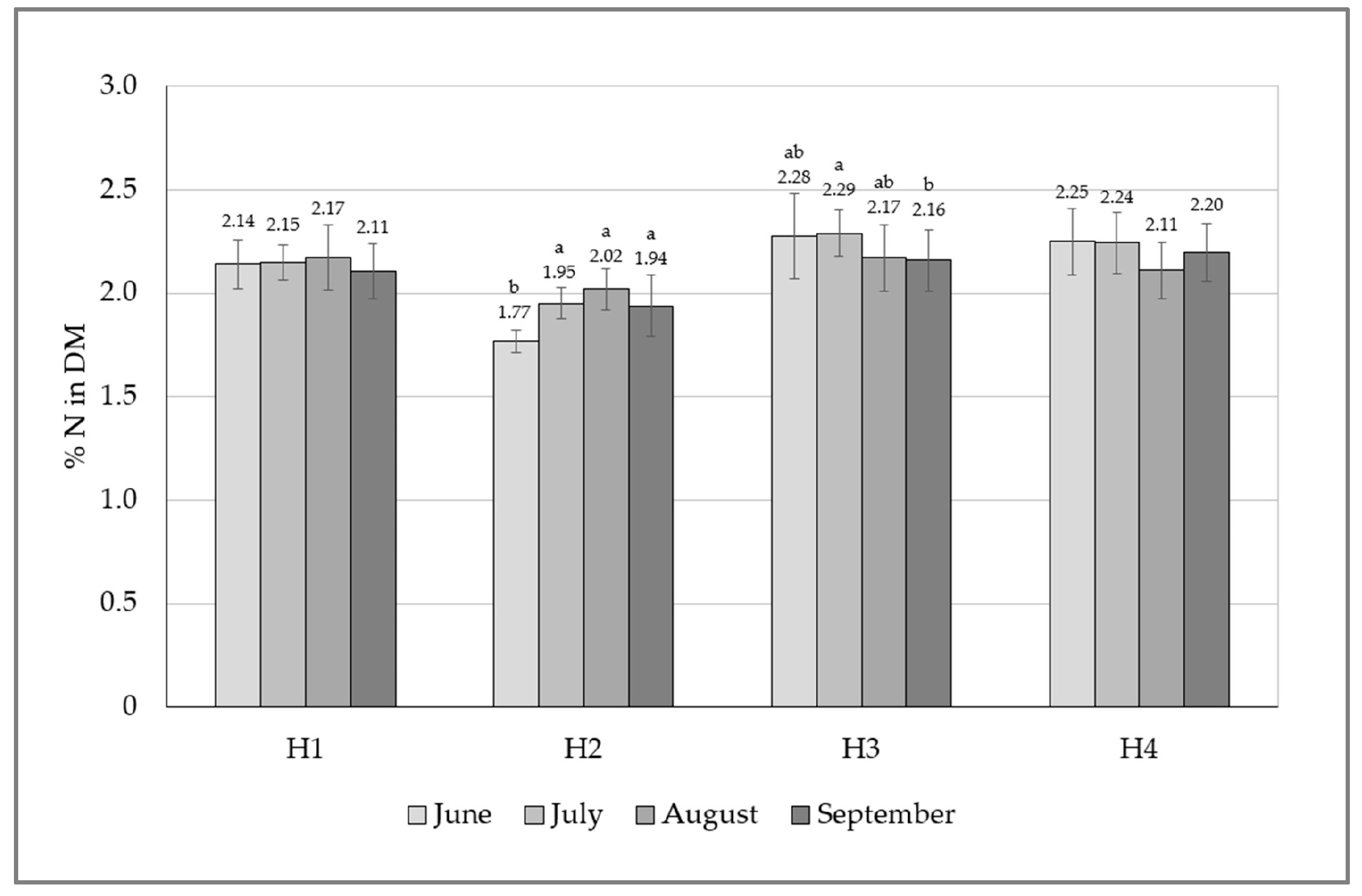
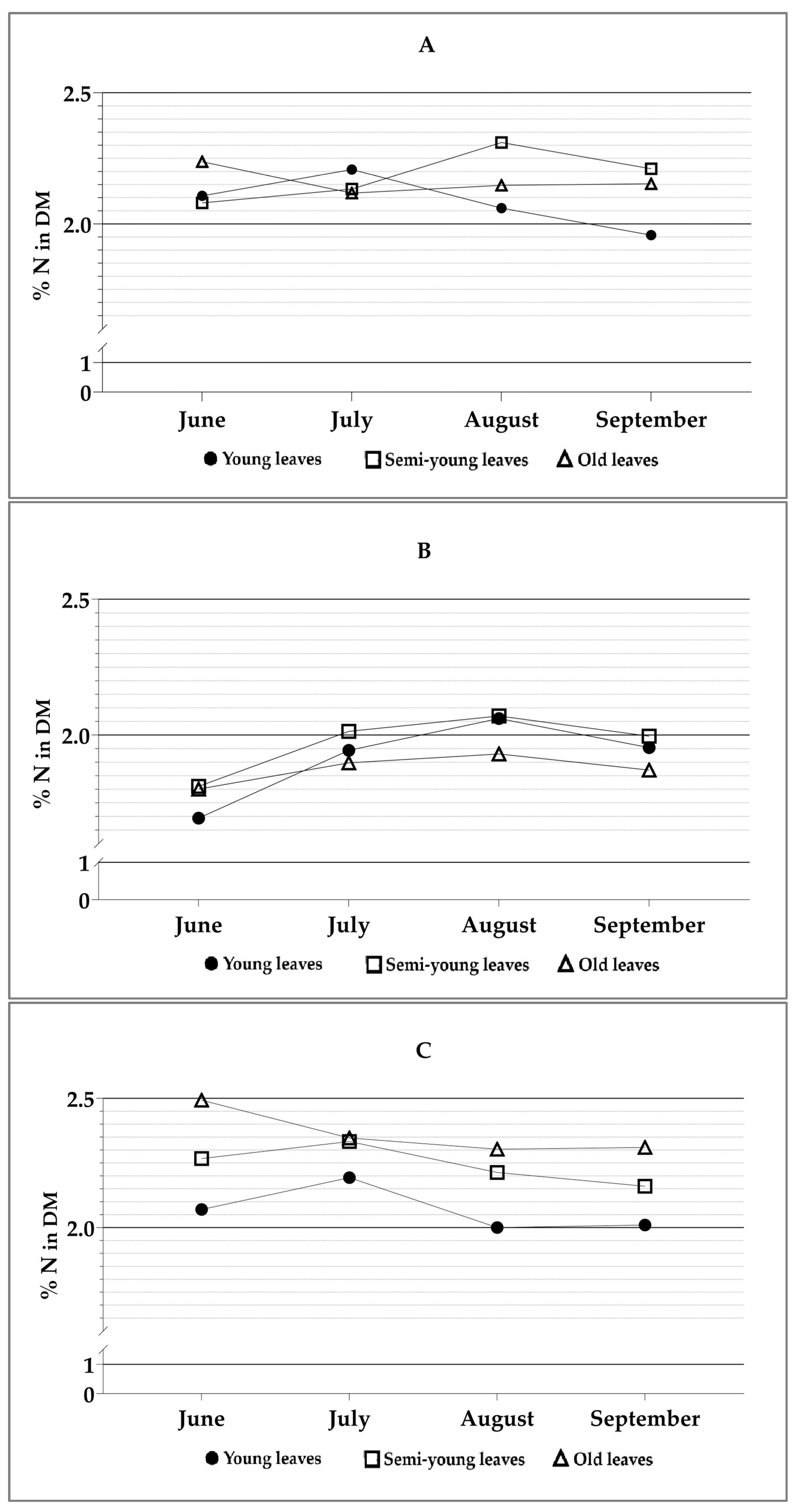
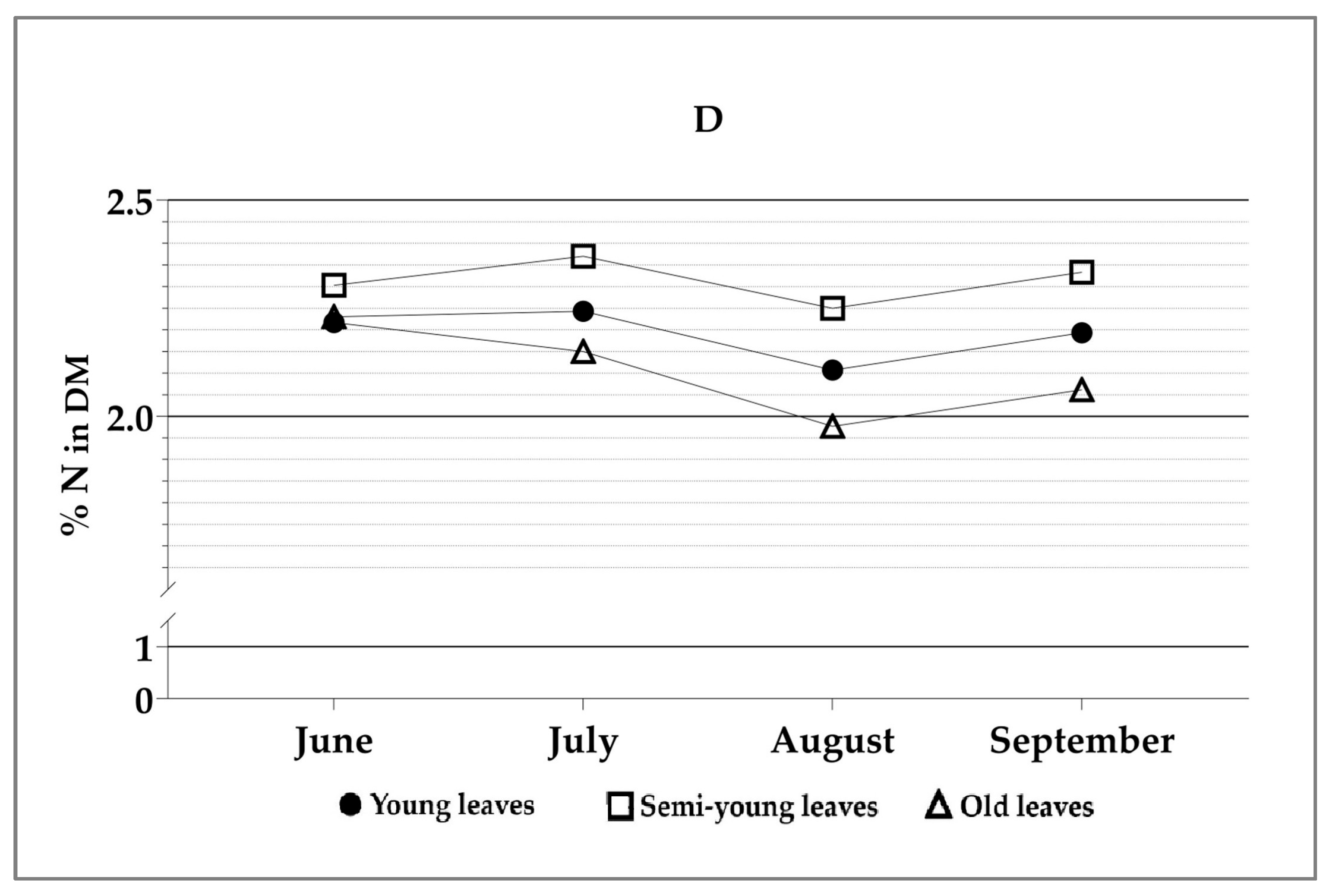
| Source | Df | H1 | H2 | H3 | H4 |
| p Value | |||||
| Age | 2.24 | 0.054 | 0.065 | <0.001 | 0.001 |
| Sampling moment | 3.24 | 0.586 | <0.001 | 0.013 | 0.073 |
| Age × sampling moment | 6.24 | 0.022 | 0.547 | 0.367 | 0.878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šokec, A.; Fruk, G.; Vrbančić, M.Š.; Konopka, K.; Karažija, T.; Petek, M. Influence of Fertigation Regimes on Nitrogen Concentration in Apple (Malus × domestica Borkh.) Leaves at Different Age Stages. Nitrogen 2025, 6, 96. https://doi.org/10.3390/nitrogen6040096
Šokec A, Fruk G, Vrbančić MŠ, Konopka K, Karažija T, Petek M. Influence of Fertigation Regimes on Nitrogen Concentration in Apple (Malus × domestica Borkh.) Leaves at Different Age Stages. Nitrogen. 2025; 6(4):96. https://doi.org/10.3390/nitrogen6040096
Chicago/Turabian StyleŠokec, Antun, Goran Fruk, Mihaela Šatvar Vrbančić, Kristijan Konopka, Tomislav Karažija, and Marko Petek. 2025. "Influence of Fertigation Regimes on Nitrogen Concentration in Apple (Malus × domestica Borkh.) Leaves at Different Age Stages" Nitrogen 6, no. 4: 96. https://doi.org/10.3390/nitrogen6040096
APA StyleŠokec, A., Fruk, G., Vrbančić, M. Š., Konopka, K., Karažija, T., & Petek, M. (2025). Influence of Fertigation Regimes on Nitrogen Concentration in Apple (Malus × domestica Borkh.) Leaves at Different Age Stages. Nitrogen, 6(4), 96. https://doi.org/10.3390/nitrogen6040096









