The Impact of Co-Inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on Cowpea Symbiosis and Growth
Abstract
1. Introduction
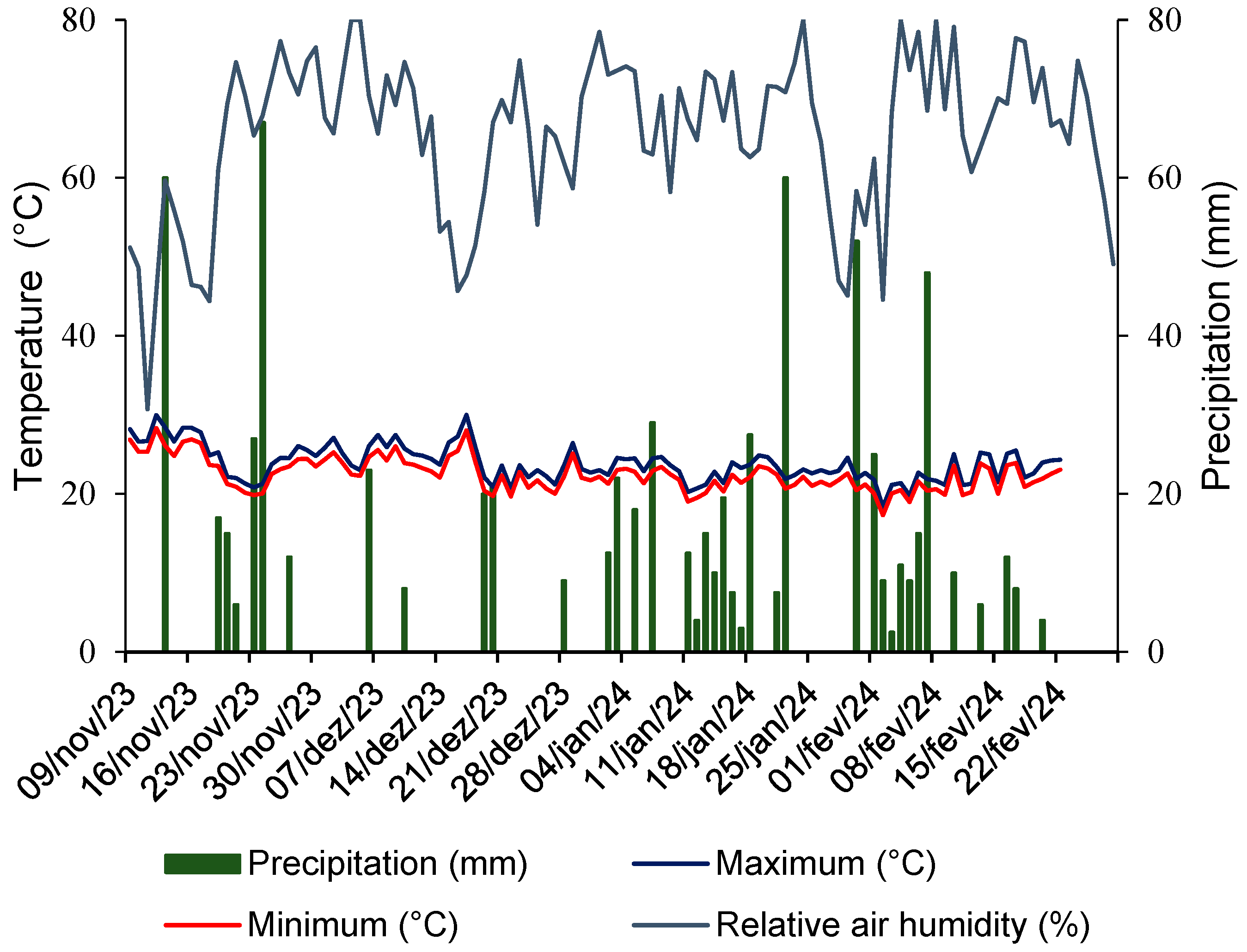
2. Materials and Methods
3. Results
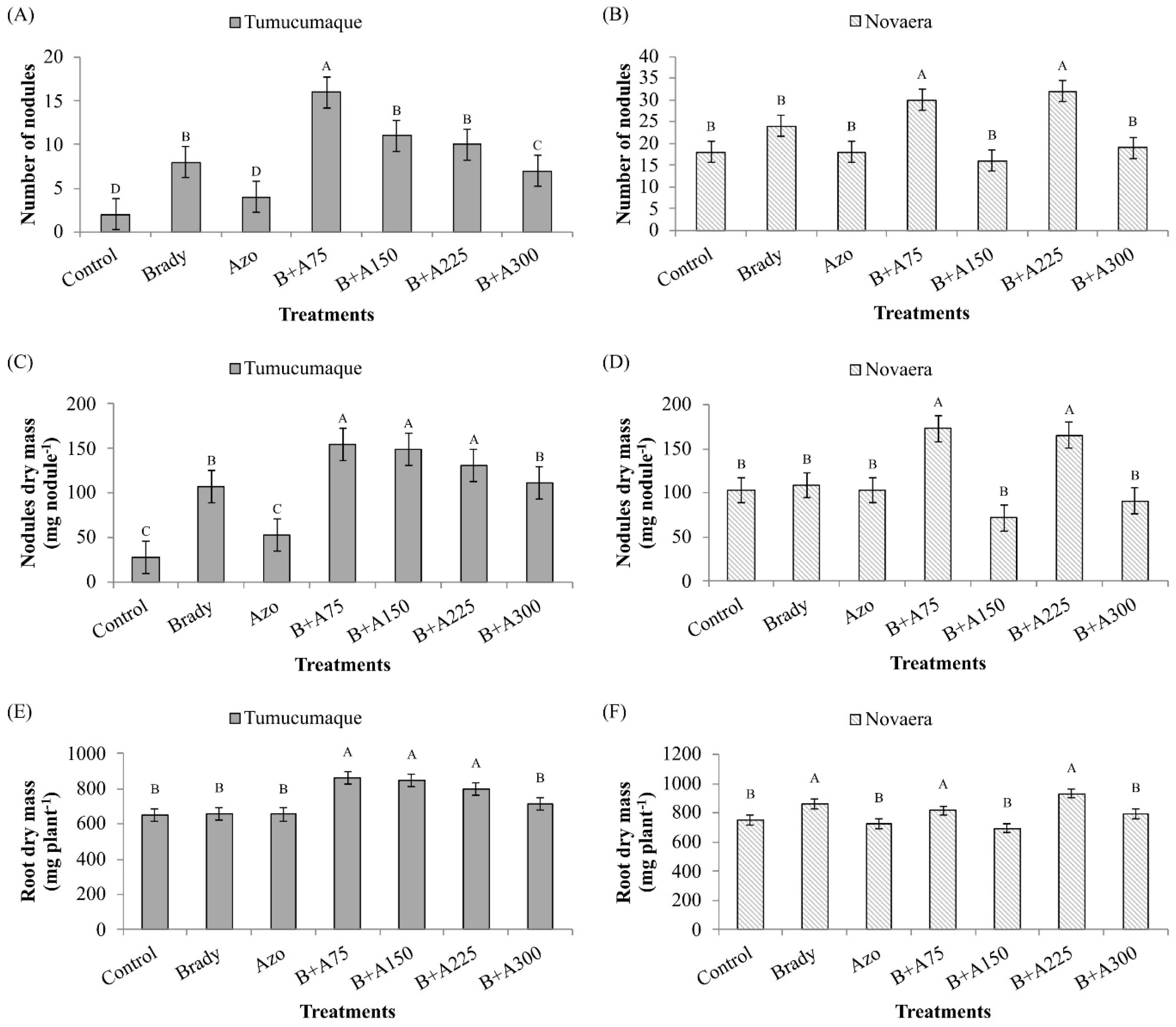
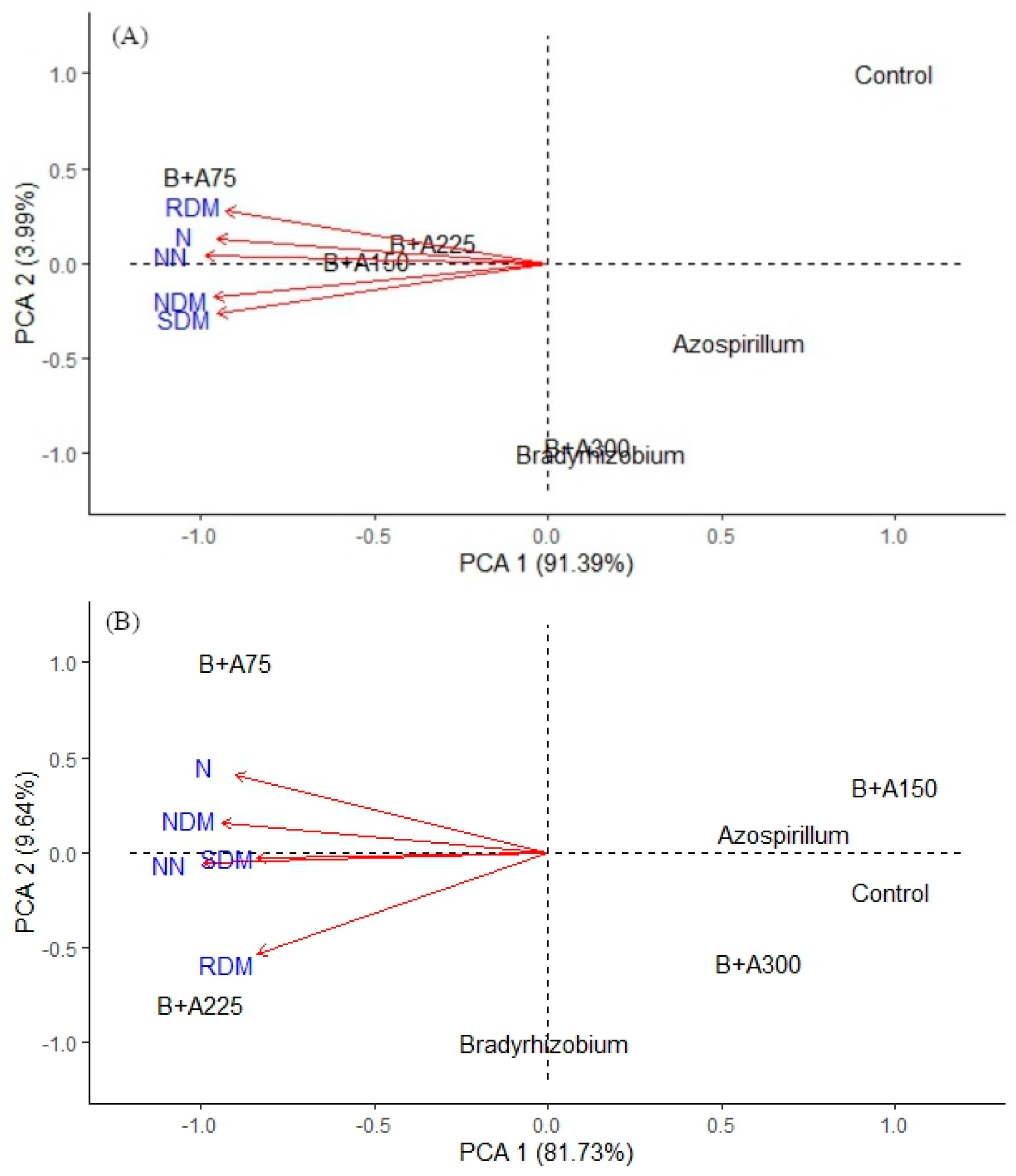
3.1. Quantification of Number of Nodules, Nodules Dry Mass and Root Dry Mass
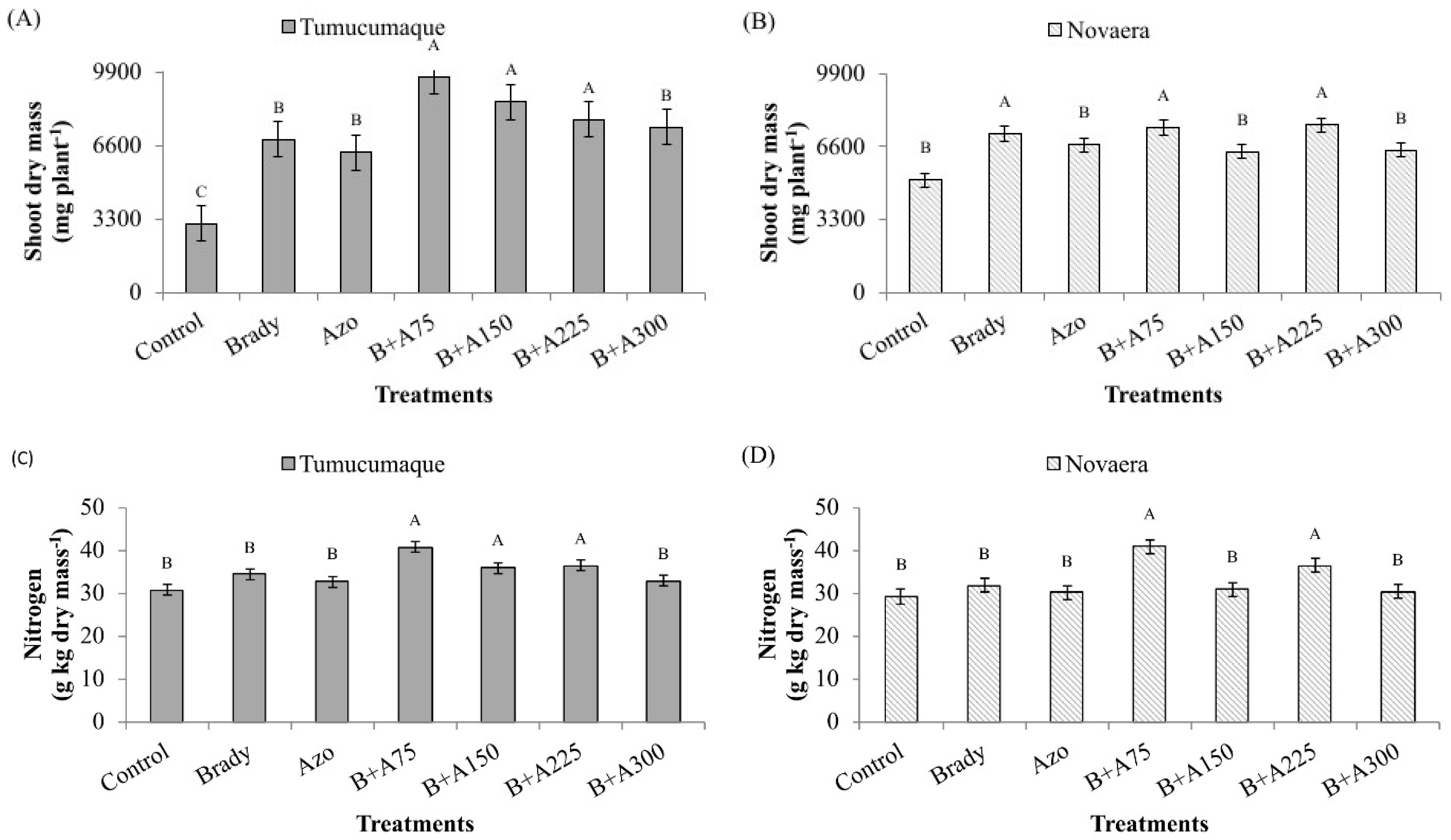
3.2. Determination of Shoot Dry Mass and Nitrogen Content
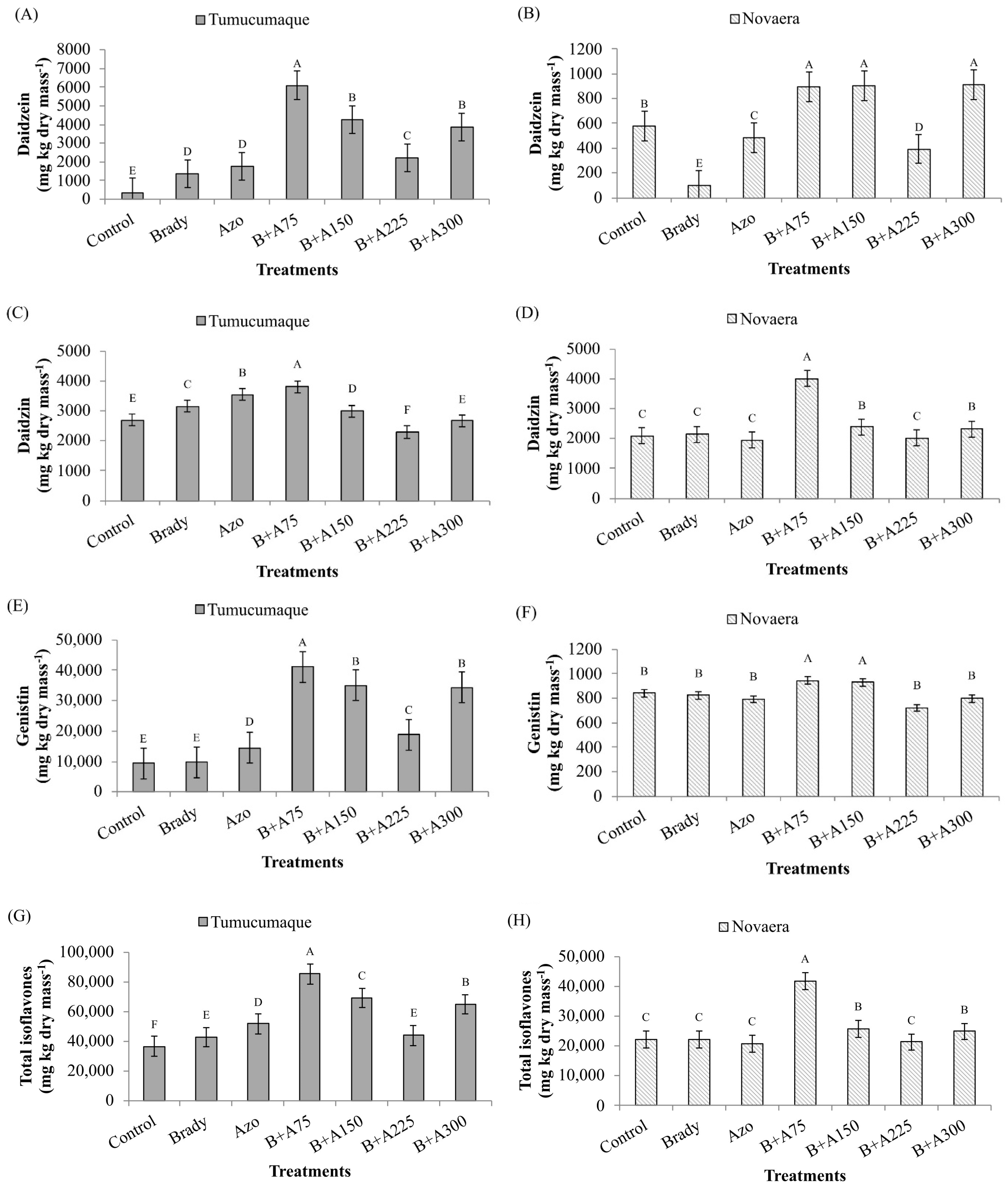
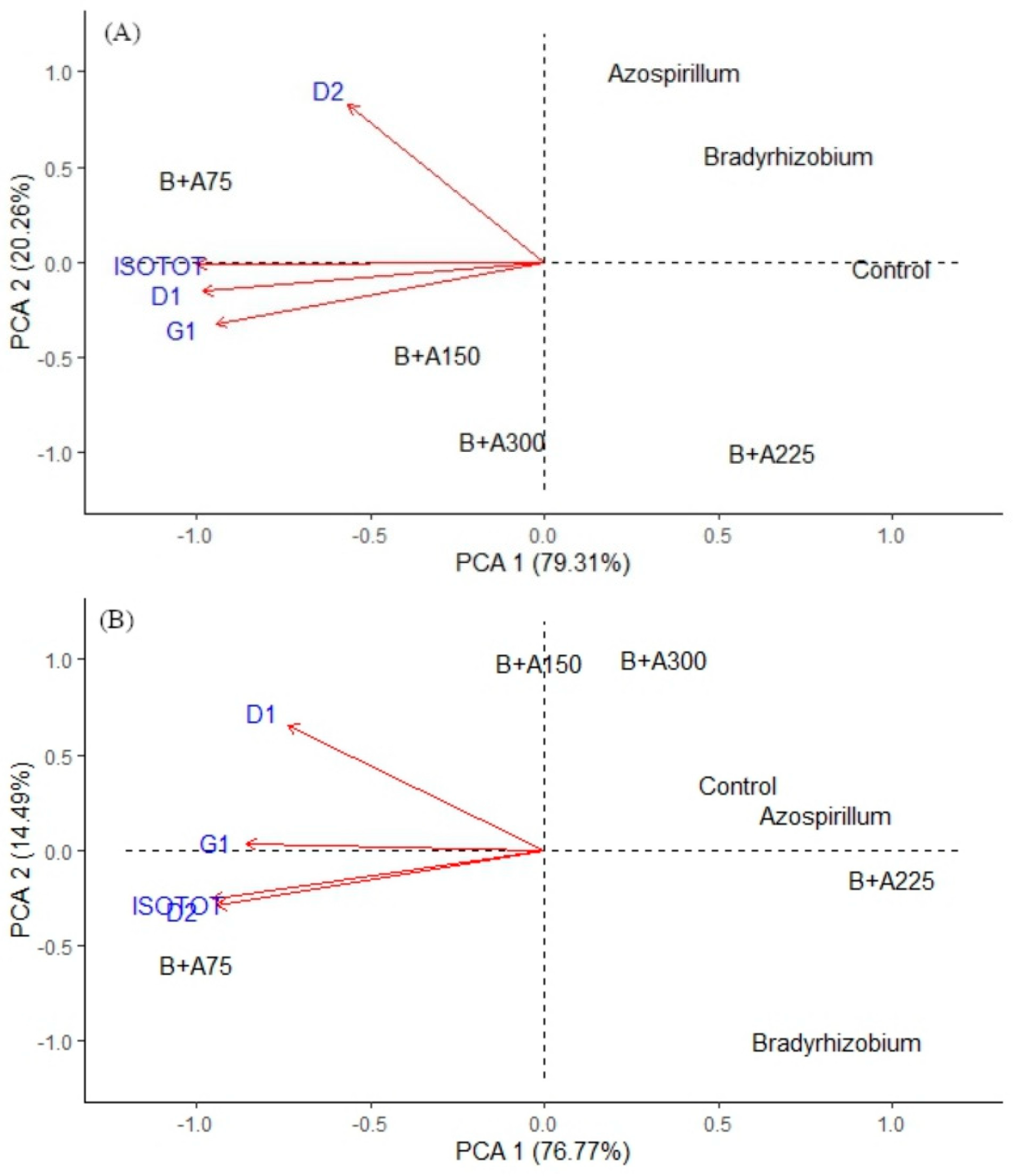
3.3. Quantification of Daidzein, Daidzin, Genistin and Total Isoflavones
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Filgueiras, G.C.; Santos, M.A.; Homma, A.K.O.; Rebello, F.K. A Cultura do Feijão-Caupi Na Amazônia Brasileira, Aspectos Socioeconômicos; Zilli, J.E., Vilarinho, A.A., Alves, J.M.A., Eds.; Embrapa Roraima: Boa Vista, Brazil, 2009; pp. 23–58. [Google Scholar]
- de Almeida, A.L.; de Alcântara, R.M.; Nóbrega, R.S.; Nóbrega, J.C.; Leite, L.F.; da Silva, J.A. Produtividade do feijão-caupi cv BR 17 Gurguéia inoculado com bactérias diazotróficas simbióticas no Piauí. Rev. Bras. Ciências Agrárias 2010, 5, 364–369. [Google Scholar] [CrossRef]
- Freire Filho, F.R.; Ribeiro, V.Q.; Rocha, M.D.; Silva, K.J.; De Nogueira, M.S.; Rodrigues, E.V. Produção, melhoramento genético e potencialidades do feijão-caupi no Brasil. Reun. Nac. Biofortif. Teresina Piauí Brasil. 2011, 4, 1–21. [Google Scholar]
- Rego, C.H.Q.; Cardoso, F.B.; Cândido, A.C.S.; Teodoro, P.E.; Alves, C.A. Co-inoculation with Bradyrhizobium and Azospirillum Increases Yield and Quality of Soybean Seeds. Agron. J. 2018, 110, 2302–2309. [Google Scholar] [CrossRef]
- Souza RAd Hungria, M.; Franchini, J.C.; Maciel, C.D.; Campo, R.J.; Zaia, D.A.M. Conjunto mínimo de parâmetros para avaliação da microbiota do solo e da fixação biológica do nitrogênio pela soja. Pesqui. Agropecuária Bras. 2008, 43, 83–91. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. ISDMct of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Bruijn, F.J.d. Biological Nitrogen Fixation; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 6, pp. 1089–1101. [Google Scholar] [CrossRef]
- Chibeba, A.M.; Guimarães, M.d.F.; Brito, O.R.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Co-inoculation of soybean with Bradyrhizobium and Azospirillum promotes early nodulation. Am. J. Plant Sci. 2015, 6, 1641–1649. [Google Scholar] [CrossRef]
- Costa, E.M.; d Carvalho, F.; d Esteves, J.A.; Nóbrega, R.S.A.; Moreira, F.M.d.S.M. Resposta da soja a inoculação e co-inoculação com bactérias promotoras do crescimento vegetal e Bradyrhizobium. Enciclopédia Biosf. 2014, 10, 1678–1689. [Google Scholar]
- Zhou, C.; Mughal, N.; Zhang, X.; Chen, J.; Shoaib, N.; Wang, X.; Yong, T.; Yang, F.; Liu, W.; Wu, Y.; et al. Soybean plants enhance growth through metabolic regulation under heterogeneous drought stress. Agric. Water Manag. 2024, 303, 109029. [Google Scholar] [CrossRef]
- Huang, X.; Chu, G.; Wang, J.; Luo, H.; Yang, Z.; Sun, L.; Rong, W.; Wang, M. Integrated metabolomic and transcriptomic analysis of specialized metabolites and isoflavonoid biosynthesis in Sophora alopecuroides L. under different degrees of drought stress. Ind. Crops Prod. 2023, 197, 116595. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, H.; Tan, F.; Wu, B.; Liu, L.; Qin, H.; Yang, Z.; He, M. Rhizosphere metabolic cross-talk from plant-soil-microbe tapping into agricultural sustainability: Current advance and perspectives. Plant Physiol. Biochem. 2024, 210, 108619. [Google Scholar] [CrossRef]
- Dong, W.; Song, Y. The significance of flavonoids in the process of biological nitrogen fixation. Int. J. Mol. Sci. 2020, 21, 5926. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- Melo, F.B.; Cardoso, M.J.; Bastos, E.A.; Ribeiro, V.Q. Recomendação de adubação e calagem para o feijão-caupi na região Meio-Norte do Brasil. Emprapa Meio Norte 2018, 1, 1–8. [Google Scholar]
- Prado, R.M. Mineral Nutrition of Tropical Plants, 1st ed.; Springer: Cham, Switzerland, 2021; p. 339. [Google Scholar]
- Carrão-Panizzi, M.C.; Favoni, S.P.d.G.; Kikuchi, A. Extraction time for isoflavone determination. Bras. Arch. Biol. Technol. 2002, 45, 515–518. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Ulzen, J.; Abaidoo, R.C.; Mensah, N.E.; Masso, C.; AbdelGadir, A.H. Bradyrhizobium Inoculants Enhance Grain Yields of Soybean and Cowpea in Northern Ghana. Plant Sci. 2016, 7, 1770. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, T.; Yoseph, T.; Högy, P.; Cadisch, G. Leaf growth, gas exchange and assimilation performance of cowpea varieties in response to Bradyrhizobium inoculation. Heliyon 2022, 8, e08746. [Google Scholar] [CrossRef] [PubMed]
- da Silva Almeida, A.; Rossetti, C.; Fleck da Rosa, G.; Moreno Rodrigues, E.; Madruga de Tunes, L.V. Biological inputs, more economy and greater sustainability. Colloq. Agrar. 2023, 18, 53–60. [Google Scholar] [CrossRef]
- Prando, A.M.; Barbosa, J.Z.; de Oliveira, A.B.; Nogueira, M.A.; Possamai, E.J.; Hungria, M. Benefits of soybean co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: Large-scale validation with farmers in Brazil. Eur. J. Agron. 2024, 155, e127112. [Google Scholar] [CrossRef]
- de Almeida Leite, R.; Martins, L.C.; Ferreira, L.V.; Barbosa, E.S.; Alves, B.J.; Zilli, J.E.; Araújo, A.P.; da Conceição Jesus, E. Co-inoculation of Rhizobium and Bradyrhizobium promotes growth and yield of common beans. Appl. Soil Ecol. 2022, 172, 104356. [Google Scholar] [CrossRef]
- Nevhulaudzi, T.; Kanu, S.A.; Ntushelo, K. Interaction effect of Bacillus subtilis co-inoculation and mine water irrigation on cowpea’s growth, physiology and nutritional quality. Sci. Afr. 2020, 9, e00541. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Xu, P.; Wang, E. Diversity and regulation of symbiotic nitrogen fixation in plants. Curr. Biol. Cell Press 2023, 33, 543–559. [Google Scholar] [CrossRef]
- Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. Flavonoids released naturally from alfalfa seeds enhance growth rate of Rhizobium meliloti. Plant Physiol. 1991, 95, 797–803. [Google Scholar] [CrossRef]
- Van Noorden, G.E.; Ross, J.J.; Reid, J.B.; Rolfe, B.G.; Mathesius, U. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 2006, 140, 1494–1506. [Google Scholar] [CrossRef]
- Bosse, M.A.; de Carvalho Mendes, N.A.; Vicente, E.F.; Tezzoto, T.; dos Reis, A.R. Nickel echances daidzein biosynthesis in roots increasing nodulation, biological nitrogen fixation and seed yeld of soybean plants. Environ. Exp. Bot. 2024, 220, 105685. [Google Scholar] [CrossRef]
- Silva, V.M.; Lui, A.C.W.; de Carvalho, M.R.; Namorato, F.A.; Fei, Z.; dos Reis, A.R.; Liu, J.; Vatamaniuk, O.K.; Li, L. The selenium-promoted daidzein production contributes to its induced nodulation in soybean plants. Environ. Exp. Bot. 2024, 218, 105591. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Alleviating salt stress on soybean (Glycine max (L.) Merr.)—Bradyrhizobium japonicum symbiosis, using signal molecule genistein. Eur. J. Soil Biol 2009, 45, 146–152. [Google Scholar] [CrossRef]
- Mai, C.; Zhao, X.; Li, X.; Wang, X.; Du, W.; Kong, Z.; Wang, L. Integrated metabolomic and transcriptomic assisted identification of nodulated Sophora flavescens SfIF7GT for improvement of flavonoid content. Ind. Crops Prod. 2024, 220, 119194. [Google Scholar] [CrossRef]
- Galatro, A.; Mas, A.L.; Luquet, M.; Fraga, C.G.; Galleano, M. Plants as a source of dietary bioactives: Flavonoids and basis for their health benefits. Asp. Mol. Med. 2024, 4, 100048. [Google Scholar] [CrossRef]
- Karuwal, R.L.; Sinay, H.; Sangur, K.; Purwaningrahayu, R.D.; Yusnawan, E.; Nugraha, Y. Identification of metabolite profiles of local cowpeas (Vigna unguiculata L. Walp) from Southwest Maluku, Indonesia. J. Agric. Food Res. 2023, 14, 100788. [Google Scholar] [CrossRef]
- Garcia, T.V.; Knaak, N.; Fiuza, L.M. Bactérias endofíticas como agentes de controle biológico na orizicultura. Arq. Inst. Biológico 2015, 82, 1–9. [Google Scholar] [CrossRef]
- Liu, C.W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere bacterial interactions and iSDMct on plant health. Curr. Opin. Microbiol. 2023, 73, 10297. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Antunes, J.E.L.; de Medeiros, V.V.; de França Barros, B.G. Resposta da co-inoculação de bactérias promotoras de crescimento em plantas e Bradyrhizobium sp. em caupi. Biosci. J. 2012, 28, 196–202. [Google Scholar]
- Da Silva, V.N.; da Silva, L.E.; de Souza, F.; Figueiredo, M.V.B. Atuação de rizóbios com rizobactéria promotora de crescimento em plantas na cultura do caupi (Vigna unguiculata (L.) Walp). Acta Sci. Agron. 2006, 28, 407–412. [Google Scholar] [CrossRef]
| Depth cm | pH CaCl2 | Al | Ca + Mg | P | K | SB | CTC | OM | V | m |
|---|---|---|---|---|---|---|---|---|---|---|
| cmolc·dm−3 | mg·dm−3 | cmolc·dm−3 | g·dm−3 | % | ||||||
| 0–20 | 5.0 | 0.07 | 4.10 | 21.9 | 99 | 4.35 | 8.4 | 26.4 | 51.5 | 1.6 |
| 20–40 | 4.7 | 0.09 | 2.00 | 5.1 | 83 | 2.21 | 6.9 | 19.8 | 32 | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontes, L.E.d.M.F.; Penha, G.C.G.; Cândido, A.C.d.S.; Campos, C.N.S.; Dutra, A.S.; Pereira, M.D.; Alves, C.Z. The Impact of Co-Inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on Cowpea Symbiosis and Growth. Nitrogen 2025, 6, 94. https://doi.org/10.3390/nitrogen6040094
Fontes LEdMF, Penha GCG, Cândido ACdS, Campos CNS, Dutra AS, Pereira MD, Alves CZ. The Impact of Co-Inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on Cowpea Symbiosis and Growth. Nitrogen. 2025; 6(4):94. https://doi.org/10.3390/nitrogen6040094
Chicago/Turabian StyleFontes, Luiz Eduardo de Morais Fernandes, Guilherme Cristyan Garcia Penha, Ana Carina da Silva Cândido, Cid Naudi Silva Campos, Alek Sandro Dutra, Márcio Dias Pereira, and Charline Zaratin Alves. 2025. "The Impact of Co-Inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on Cowpea Symbiosis and Growth" Nitrogen 6, no. 4: 94. https://doi.org/10.3390/nitrogen6040094
APA StyleFontes, L. E. d. M. F., Penha, G. C. G., Cândido, A. C. d. S., Campos, C. N. S., Dutra, A. S., Pereira, M. D., & Alves, C. Z. (2025). The Impact of Co-Inoculation with Bradyrhizobium japonicum and Azospirillum brasilense on Cowpea Symbiosis and Growth. Nitrogen, 6(4), 94. https://doi.org/10.3390/nitrogen6040094









