Biofertilizers for Enhanced Nitrogen Use Efficiency: Mechanisms, Innovations, and Challenges
Abstract
1. Introduction
2. Agrotechnical Methods for Increasing the Efficiency of Fertilizer Nitrogen Use
- Adoption of precision agriculture tools. Remote sensing, soil and crop sensors, and variable-rate technology allow site-specific adjustment of N rates according to spatial variability and temporal changes in crop N status. Proximal and remote sensing of canopy reflectance or chlorophyll (e.g., SPAD readings, multispectral indices) can be used to detect N deficiencies early and refine in-season N recommendations [20]. This reduces the common tendency to apply uniform and often excessive N rates as yield “insurance” and thus contributes to higher NUE and lower environmental losses [21].
- Optimization of application timing. Splitting N applications and targeting critical phenological stages improves synchrony between N supply and crop demand [22]. By postponing part of the N dose to periods of rapid biomass accumulation, the residence time of mineral N in the soil is reduced, lowering the risk of leaching, volatilization and denitrification. In many cropping systems, such temporal optimization allows total N inputs to be decreased without penalizing yield, and may even improve grain or fruit quality [23].
- Nitrogen rate calculation based on crop demand and nutrient removal. Accurate nitrogen fertilizer rates should be calculated from crop-specific N uptake curves, expected yield, and nutrient removal coefficients, rather than from conventional fixed rates or farmer intuition. Evidence shows that N is often applied in excess due to “insurance” practices rather than actual crop requirements, leading to low recovery efficiency and higher losses. Rigorous N budgeting; including soil residual N, mineralization estimates, and realistic yield goals; is essential for aligning supply with plant demand [24,25].
- Smart fertilizers (controlled-release and inhibitor-enhanced) to reduce volatilization and leaching. Smart N fertilizers, including controlled-release products and urea treated with urease or nitrification inhibitors, improve nitrogen use efficiency by synchronizing N release with crop uptake and reducing loss pathways such as ammonia volatilization and nitrate leaching. Meta-analyses show significant reductions in NH3 losses and NO3− leaching compared with conventional urea, demonstrating their value as an agrotechnical method for efficient N management [26,27].
- Improved fertilizer placement. Incorporating N into the soil or applying it in bands close to the seed or root zone enhances contact between fertilizer granules and active roots [28]. Compared with surface broadcasting, these strategies reduce ammonia volatilization, limit immobilization in surface residues and create localized zones of high N availability that stimulate root proliferation. The integration of banded placement with localized irrigation (e.g., drip lines near N bands) can further improve N recovery by crops [29].
- Enhancement of soil health. Practices such as diversified crop rotations, the use of cover crops and the addition of organic amendments (manure, compost, crop residues) increase soil organic matter and improve soil structure, porosity and water-holding capacity [30]. A more biologically active soil supports microbial processes that regulate N cycling, including mineralization, immobilization and nitrification, thereby enhancing N retention within the system. Over the medium term, these improvements in soil health contribute to more stable yields with lower external N inputs [31].
- Improved water management and fertigation. Precise irrigation scheduling and technologies such as drip irrigation or subsurface drip systems help maintain soil water content within an optimal range, minimizing N leaching and runoff [32]. When combined with fertigation, water and dissolved N fertilizers can be applied frequently in small doses, closely matching crop water and N requirements in space and time. This approach has shown substantial potential to increase NUE, while simultaneously reducing N losses and mitigating environmental impacts. Controlled drainage or deficit-irrigation strategies can also be used to further limit N transport to groundwater and surface waters [33].
- Use of high-efficiency crop varieties. Cultivars selected for improved root architecture, higher N uptake capacity, or enhanced N assimilation and remobilization efficiency can achieve similar or higher yields at reduced N rates. Such genotypes often display greater resilience under sub-optimal N supply and may be particularly effective when combined with precision N management and improved soil and water practices. Integrating genetic and management approaches thus offers a promising pathway to further increase NUE at the cropping-system scale [34].
3. Mechanism for Nitrogen Use Efficient Improvement
3.1. Nutrient Mobilization: Enhanced Solubilization of Bound Nutrients Improves Nitrogen Uptake Synergy
3.2. Nutrient-Cycling Regulation: Suppressing Nitrification or Denitrification Reduces Nitrogen Losses
3.3. Root Growth Stimulation: Larger and Healthier Roots Improve Nutrient Absorption
3.4. Stress Mitigation: Biofertilizers Help Plants Cope with Abiotic Stress (Salinity, Drought), Maintaining Nitrogen Metabolism Efficiency
4. Biological Nitrogen Fixation
4.1. Symbiotic: Rhizobium–Legume Interactions, Bradyrhizobium, Sinorhizobium and Cyanobacteria
4.1.1. Rhizobium–Legume System
4.1.2. Bradyrhizobium–Soybean System
4.1.3. Sinorhizobium–Medicago System
4.1.4. Cyanobacterial Nitrogen Fixation in Rice Systems
4.2. Associative: Azospirillum, Herbaspirillum, Methylobacterium, Bacillus
4.2.1. Azospirillum: A Model Associative Diazotroph
4.2.2. Herbaspirillum: Endophytic Diazotrophs in Grasses
4.2.3. Methylobacterium: Pink Phyllospheric Partners
4.2.4. Bacillus: Versatile PGPR with Nitrogen-Fixing Ability
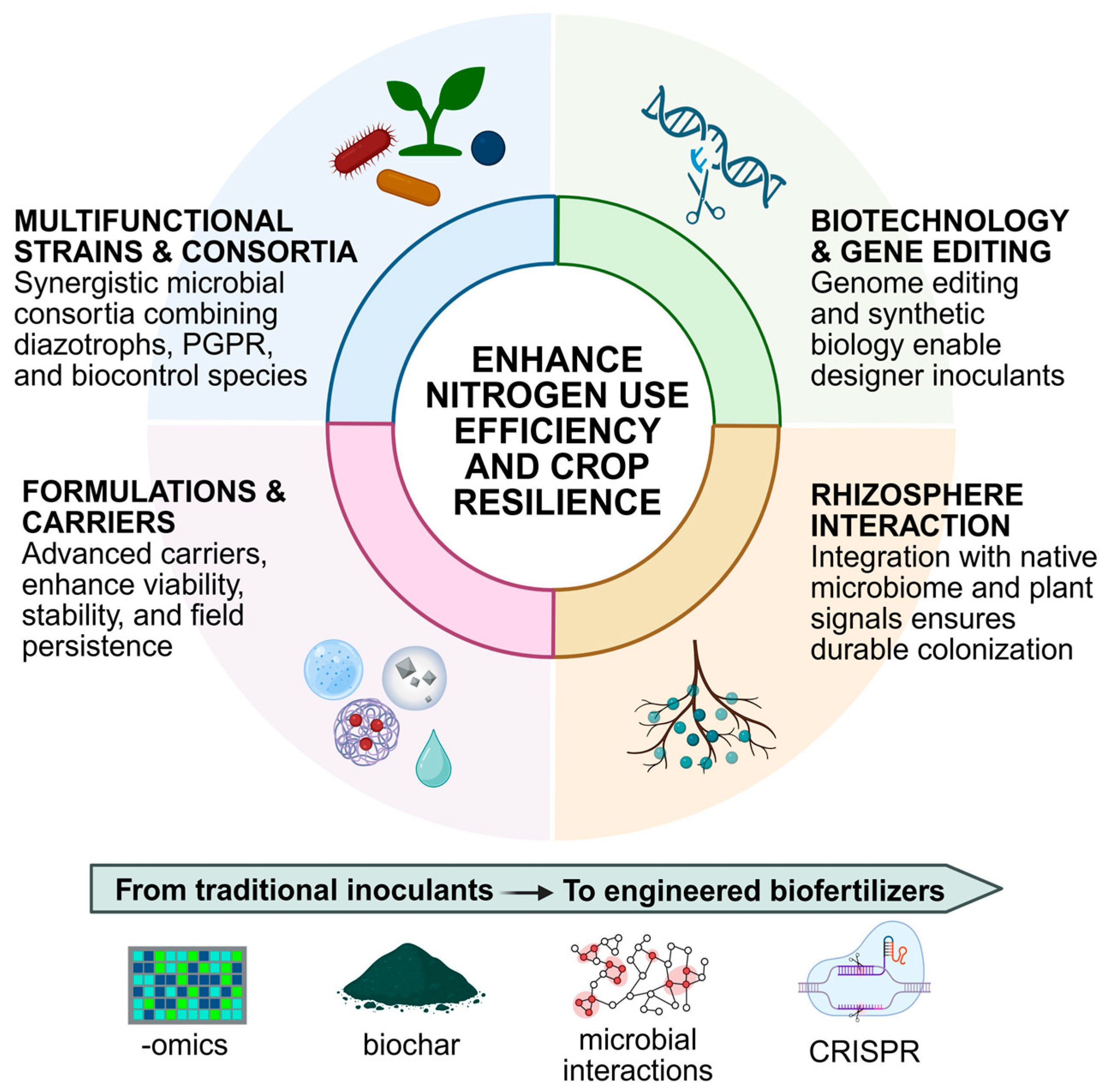
5. Recent Advances in Biofertilizer Development
5.1. Multifunctional Strains and Microbial Consortia
5.2. Formulation and Carriers
5.3. Interaction with the Soil and Rhizosphere Microbiota
5.4. Biotechnology and Gene Editing
6. Bottlenecks and Challenges
7. Market Landscape and Future Perspectives
8. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The Utilization and Roles of Nitrogen in Plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; Sujayanand, G.K.; Vadivel, R.; Das, T.K.; et al. Nitrogen Use Efficiency—A Key to Enhance Crop Productivity under a Changing Climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing Nitrogen for Sustainable Development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Davidson, E.A.; Kanter, D. Inventories and Scenarios of Nitrous Oxide Emissions. Environ. Res. Lett. 2014, 9, 105012. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global Agriculture and Nitrous Oxide Emissions. Nat. Clim. Change 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Rosa, L.; Gabrielli, P. Energy and Food Security Implications of Transitioning Synthetic Nitrogen Fertilizers to Net-Zero Emissions. Environ. Res. Lett. 2023, 18, 014008. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Global Nitrogen Fertiliser Demand and Supply: Trend, Current Level and Outlook Figure 1: Evolution of Global Fertilizer Consumption by Nutrient (Tg N+P2O5+K2O) (IFA, 2016a). In Proceedings of the 2016 International Nitrogen Initiative Conference, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K. Zothanpuia Plant Growth Promoting Bacteria (PGPB)-Induced Plant Adaptations to Stresses: An Updated Review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef]
- Liu-Xu, L.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Scalschi, L.; Llorens, E. Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture. Horticulturae 2024, 10, 621. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sustainable Nitrogen Management in Agrifood Systems|FAO. Available online: https://www.fao.org/family-farming/detail/en/c/1732256/ (accessed on 5 October 2025).
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Osborne, S.L.; Schepers, J.S.; Francis, D.D.; Schlemmer, M.R. Use of Spectral Radiance to Estimate In-Season Biomass and Grain Yield in Nitrogen- and Water-Stressed Corn. Dep. Agron. Hortic. Fac. Publ. 2002, 42, 165–171. [Google Scholar]
- Mulla, D.J. Twenty Five Years of Remote Sensing in Precision Agriculture: Key Advances and Remaining Knowledge Gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Ancev, T.; Bostian, M. Optimal Timing and Rate of Nitrogen Fertilizer Use: An Integrated Network Technology Approach. Am. J. Agric. Econ. 2025, 1–18. [Google Scholar] [CrossRef]
- Hu, Y. Improving Nitrogen Use Efficiency for Corn (Zea mays L.) Production. Front. Sci. Eng. 2023, 3, 57–61. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving Nitrogen Use Efficiency for Cereal Production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Dobermann, A. Nutrient Use Efficiency: Measurement and Management. In Fertilizer Best Management Practices: General Principles, Strategy for Their Adoption and Voluntary Initiatives Versus Regulations; International Fertilizer Industry Association: Paris, France, 2007. [Google Scholar]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 3rd ed.; IFA: Paris, France, 2010; pp. 2–99. [Google Scholar]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of Effectiveness of Enhanced-Efficiency Fertilizers as Mitigation Options for N2O and NO Emissions from Agricultural Soils: Meta-Analysis. Glob. Change Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer Placement to Improve Crop Nutrient Acquisition and Yield: A Review and Meta-Analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Z.; Du, L.; Sun, G.; Su, L.; Xiao, Z.; Li, C.; Wang, Z.; Xiao, Z.; Hu, T.; et al. Effect of Deep Placement of Large Granular Fertilizer on Ammonia Volatilization, Soil Nitrogen Distribution and Rice Growth. Agronomy 2022, 12, 2066. [Google Scholar] [CrossRef]
- Westerik, D.; Hoffland, E.; Hijbeek, R. Nitrogen Fertilizer Replacement Values of Organic Amendments: Determination and Prediction. Nutr. Cycl. Agroecosyst. 2023, 129, 445–458. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Health and Sustainability: Managing the Biotic Component of Soil Quality Soil Quality Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Wu, Y.; Si, W.; Yan, S.; Wu, L.; Zhao, W.; Zhang, J.; Zhang, F.; Fan, J. Water Consumption, Soil Nitrate-Nitrogen Residue and Fruit Yield of Drip-Irrigated Greenhouse Tomato under Various Irrigation Levels and Fertilization Practices. Agric. Water Manag. 2023, 277, 108092. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Liang, H.; Hu, K.; Qin, S.; Guo, H. Comparison of Water-and Nitrogen-Use Efficiency over Drip Irrigation with Border Irrigation Based on a Model Approach. Agronomy 2020, 10, 1890. [Google Scholar] [CrossRef]
- Cormier, F.; Foulkes, J.; Hirel, B.; Gouache, D.; Moënne-Loccoz, Y.; Le Gouis, J. Breeding for Increased Nitrogen-Use Efficiency: A Review for Wheat (T. Aestivum L.). Plant Breed. 2016, 135, 255–278. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil. 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Leidi, E.O.; RodrÍGUEZ-NAVARRO, D.N. Nitrogen and Phosphorus Availability Limit N2 Fixation in Bean. New Phytol. 2000, 147, 337–346. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Spychaj-Fabisiak, E.; Pior, N. Prediction of Molybdenum Availability to Plants in Differentiated Soil Conditions. Plant Soil. Environ. 2017, 63, 491–497. [Google Scholar] [CrossRef]
- Symanowicz, B.; Kalembasa, S. Effect of Iron, Molybdenum and Cobalt on the Amount of Nitrogen Biologically Reduced by Rhizobium Galegae. Ecol. Chem. Eng. A 2012, 19, 1311–1320. [Google Scholar]
- Albornoz, F.; Godoy, L. Modulation of Root Nitrogen Uptake Mechanisms Mediated by Beneficial Soil Microorganisms. Plants 2025, 14, 2729. [Google Scholar] [CrossRef]
- Subbarao, G.; Ito, O.; Sahrawat, K.; Berry, W.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I. Scope and Strategies for Regulation of Nitrification in Agricultural Systems—Challenges and Opportunities. CRC Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for Biological Nitrification Inhibition in Brachiaria Pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef]
- Calvo, P.; Watts, D.B.; Ames, R.N.; Kloepper, J.W.; Torbert, H.A. Microbial-Based Inoculants Impact Nitrous Oxide Emissions from an Incubated Soil Medium Containing Urea Fertilizers. J. Environ. Qual. 2013, 42, 704–712. [Google Scholar] [CrossRef]
- Mai, W.; Chen, J.; Liu, H.; Liang, J.; Tang, J.; Wei, Y. Advances in Studies on Microbiota Involved in Nitrogen Removal Processes and Their Applications in Wastewater Treatment. Front. Microbiol. 2021, 12, 746293. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding Plant Response to Nitrogen Limitation for the Improvement of Crop Nitrogen Use Efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-Mediated Drought Tolerance: Current and Future Prospects. Appl. Soil. Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic Effect of Pseudomonas putida and Bacillus amyloliquefaciens Ameliorates Drought Stress in Chickpea (Cicer Arietinum L.). Plant Signal Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by Arbuscular Mycorrhizae of the Integrated Physiological Response to Salinity in Plants: New Challenges in Physiological and Molecular Studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.S.P. Legume Nitrogen Fixation in a Changing Environment: Achievements and Challenges; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–133. [Google Scholar] [CrossRef]
- Lindström, K.; Murwira, M.; Willems, A.; Altier, N. The Biodiversity of Beneficial Microbe-Host Mutualism: The Case of Rhizobia. Res. Microbiol. 2010, 161, 453–463. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Threatt, S.D.; Rees, D.C. Biological Nitrogen Fixation in Theory, Practice, and Reality: A Perspective on the Molybdenum Nitrogenase System. FEBS Lett. 2022, 597, 45. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The Contributions of Nitrogen-Fixing Crop Legumes to the Productivity of Agricultural Systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Reis Ely, C.R.; Perakis, S.S.; Cleveland, C.C.; Menge, D.N.L.; Reed, S.C.; Batterman, S.A.; Crews, T.E.; Dynarski, K.A.; Gei, M.; Gundale, M.J.; et al. A Global Dataset of Terrestrial Biological Nitrogen Fixation. Sci. Data 2025, 12, 1362. [Google Scholar] [CrossRef]
- Reis Ely, C.R.; Perakis, S.S.; Cleveland, C.C.; Menge, D.N.L.; Reed, S.C.; Taylor, B.N.; Batterman, S.A.; Clark, C.M.; Crews, T.E.; Dynarski, K.A.; et al. Global Terrestrial Nitrogen Fixation and Its Modification by Agriculture. Nature 2025, 643, 705–711. [Google Scholar] [CrossRef]
- Qiao, M.; Sun, R.; Wang, Z.; Dumack, K.; Xie, X.; Dai, C.; Wang, E.; Zhou, J.; Sun, B.; Peng, X.; et al. Legume Rhizodeposition Promotes Nitrogen Fixation by Soil Microbiota under Crop Diversification. Nat. Commun. 2024, 15, 2924. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Chiurazzi, M.; Frugis, G.; Navazio, L. Symbiotic Nitrogen Fixation: A Launchpad for Investigating Old and New Challenges. J. Exp. Bot. 2025, 76, 1473–1477. [Google Scholar] [CrossRef]
- Hubbell, D.H.; Gaskins, M.H. Associative N2 Fixation with Azospirillum. In Biological Nitrogen Fixation: Ecology, Technology and Physiology; Springer: Boston, MA, USA, 1984; pp. 201–224. [Google Scholar] [CrossRef]
- Smercina, D.N.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. To Fix or Not To Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere. Appl. Environ. Microbiol. 2019, 85, e02546-18. [Google Scholar] [CrossRef] [PubMed]
- Boivin, S.; Mahé, F.; Debellé, F.; Pervent, M.; Tancelin, M.; Tauzin, M.; Wielbo, J.; Mazurier, S.; Young, P.; Lepetit, M. Genetic Variation in Host-Specific Competitiveness of the Symbiont Rhizobium leguminosarum Symbiovar Viciae. Front. Plant Sci. 2021, 12, 719987. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Bueis, R.; Sánchez-Cañizares, C.; James, E.K.; González-Andrés, F. Formulation of a Highly Effective Inoculant for Common Bean Based on an Autochthonous Elite Strain of Rhizobium leguminosarum Bv. Phaseoli, and Genomic-Based Insights Into Its Agronomic Performance. Front. Microbiol. 2019, 10, 490875. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Kozieł, M.; Adamczyk, P.; Buczek, K.; Kalita, M.; Gromada, A.; Mordzińska-Rak, A.; Polakowski, C.; Bieganowski, A. Symbiotic Efficiency of Rhizobium leguminosarum Sv. Trifolii Strains Originating from the Subpolar and Temperate Climate Regions. Sci. Rep. 2024, 14, 6264. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, O.M.; Riva, O.; Peltzer, E. Analysis of Rhizobium Etli and of Its Symbiosis with Wild Phaseolus Vulgaris Supports Coevolution in Centers of Host Diversification. Proc. Natl. Acad. Sci. USA 2004, 101, 13548–13553. [Google Scholar] [CrossRef]
- Appunu, C.; Dhar, B. Symbiotic Effectiveness of Acid-Tolerant Bradyrhizobium Strains with Soybean in Low PH Soil. Afr. J. Biotechnol. 2006, 5, 842–845. [Google Scholar]
- Vinuesa, P.; León-Barrios, M.; Silva, C.; Willems, A.; Jarabo-Lorenzo, A.; Pérez-Galdona, R.; Werner, D.; Martínez-Romero, E. Bradyrhizobium canariense sp. Nov., an Acid-Tolerant Endosymbiont That Nodulates Endemic Genistoid Legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum Bv. Genistearum, Bradyrhizobium Genospecies Alpha and Bradyrhizobium Genospecies Beta. Int. J. Syst. Evol. Microbiol. 2005, 55, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zadegan, S.B.; Kim, W.; Abbas, H.M.K.; Kim, S.; Krishnan, H.B.; Hewezi, T. Differential Symbiotic Compatibilities between Rhizobium Strains and Cultivated and Wild Soybeans Revealed by Anatomical and Transcriptome Analyses. Front. Plant Sci. 2024, 15, 1435632. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, H.; Qi, X.; He, T.; Zhang, B.; Wang, E.; Yu, M.; Wang, B.; Wang, F.; Liu, Z.; et al. Diversification of Sinorhizobium Populations Associated with Medicago Polymorpha and Medicago Lupulina in Purple Soil of China. Front. Microbiol. 2023, 13, 1055694. [Google Scholar] [CrossRef] [PubMed]
- Elboutahiri, N.; Thami-Alami, I.; Udupa, S.M. Phenotypic and Genetic Diversity in Sinorhizobium meliloti and S. medicae from Drought and Salt Affected Regions of Morocco. BMC Microbiol. 2010, 10, 15. [Google Scholar] [CrossRef]
- Liu, F.; Yi, M.; Liu, X.; Shen, Y.; Li, J.; Wang, H.; Yang, D.; Sun, Z. Symbiotic Performances of Three Mesorhizobium Huakuii Strains Inoculated to Chinese Milk Vetch Varieties. Front. Plant Sci. 2020, 11, 599400. [Google Scholar] [CrossRef]
- Cooper, J.E.; Wood, M.; Bjourson, A.J. Nodulation of Lotus Pedunculatus in Acid Rooting Solution by Fast- and Slow-Growing Rhizobia. Soil. Biol. Biochem. 1985, 17, 487–492. [Google Scholar] [CrossRef]
- Osborne, B.; Bergman, B. Why Does Gunnera Do It and Other Angiosperms Don’t? An Evolutionary Perspective on the Gunnera–Nostoc Symbiosis. In Prokaryotic Symbionts in Plants. Microbiology Monographs; Pawlowski, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 8, pp. 207–224. [Google Scholar] [CrossRef]
- De Vries, S.; De Vries, J. Evolutionary Genomic Insights into Cyanobacterial Symbioses in Plants. Quant. Plant Biol. 2022, 3, e16. [Google Scholar] [CrossRef]
- Adams, D.G.; Duggan, P.S. Cyanobacteria–Bryophyte Symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef]
- Sierra, A.M.; Toupin, S.; Alonso-García, M.; Villarreal, A.J.C. Diversity of Symbiotic Cyanobacteria in Cycad Coralloid Roots Using a Short-Read RbcL-X Amplicon. Symbiosis 2024, 92, 271–288. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Ravelo-Ortega, G.; García-Pineda, E.; López-Bucio, J. A Century of Azospirillum: Plant Growth Promotion and Agricultural Promise. Plant Signal Behav. 2025, 20, 2551609. [Google Scholar] [CrossRef]
- Muthukumarasamy, R.; Govindarajan, M.; Vadivelu, M.; Revathi, G. N-Fertilizer Saving by the Inoculation of Gluconacetobacter diazotrophicus and Herbaspirillum sp. in Micropropagated Sugarcane Plants. Microbiol. Res. 2006, 161, 238–245. [Google Scholar] [CrossRef]
- Walitang, D.I.; Roy Choudhury, A.; Lee, Y.; Choi, G.; Jeong, B.; Jamal, A.R.; Sa, T. The Endophytic Plant Growth Promoting Methylobacterium Oryzae CBMB20 Integrates and Persists into the Seed-Borne Endophytic Bacterial Community of Rice. Agriculture 2023, 13, 355. [Google Scholar] [CrossRef]
- Jain, S.; Varma, A.; Choudhary, D.K. Perspectives on Nitrogen-Fixing Bacillus Species. In Soil Nitrogen Ecology; Springer: Cham, Switzerland, 2021; pp. 359–369. [Google Scholar] [CrossRef]
- Galardini, M.; Mengoni, A.; Brilli, M.; Pini, F.; Fioravanti, A.; Lucas, S.; Lapidus, A.; Cheng, J.F.; Goodwin, L.; Pitluck, S.; et al. Exploring the Symbiotic Pangenome of the Nitrogen-Fixing Bacterium Sinorhizobium meliloti. BMC Genom. 2011, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.; Branca, A.; Mudge, J.; Bharti, A.K.; Briskine, R.; Farmer, A.D.; Sugawara, M.; Young, N.D.; Sadowsky, M.J.; Tiffin, P. Population Genomics of the Facultatively Mutualistic Bacteria Sinorhizobium meliloti and S. medicae. PLoS Genet. 2012, 8, e1002868. [Google Scholar] [CrossRef]
- Ghantasala, S.; Roy Choudhury, S. Nod Factor Perception: An Integrative View of Molecular Communication during Legume Symbiosis. Plant Mol. Biol. 2022, 110, 485–509. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Fujishige, N.A. Molecular Signals and Receptors: Communication Between Nitrogen-Fixing Bacteria and Their Plant Hosts. In Biocommunication of Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 255–280. [Google Scholar] [CrossRef]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-Mediated Exopolysaccharide Perception Controls Bacterial Infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef]
- de Carvalho-Niebel, F.; Fournier, J.; Becker, A.; Marín Arancibia, M. Cellular Insights into Legume Root Infection by Rhizobia. Curr. Opin. Plant Biol. 2024, 81, 102597. [Google Scholar] [CrossRef]
- Kalembasa, D.; Szukała, J.; Symanowicz, B.; Kalembasa, S.; Faligowska, A.; Becher, M. Amount of Biologically Nitrogen Fixed by Faba Bean and Its Uptake by Winter Wheat Determined by 15N ID Method. Arch. Agron. Soil. Sci. 2020, 67, 1875–1888. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; Saidi, M.; Belhadj, O.; Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; et al. Potential of Rhizobia in Improving Nitrogen Fixation and Yields of Legumes. In Symbiosis; Books on Demand: Norderstedt, Germany, 2018. [Google Scholar] [CrossRef]
- Serafin-Andrzejewska, M.; Falkiewicz, A.; Wojciechowski, W.; Kozak, M. Yield and Seed Quality of Faba Bean (Vicia faba L. var. minor) as a Result of Symbiosis with Nitrogen-Fixing Bacteria. Agriculture 2025, 15, 960. [Google Scholar] [CrossRef]
- Lira, M.A.; Nascimento, L.R.S.; Fracetto, G.G.M. Legume-Rhizobia Signal Exchange: Promiscuity and Environmental Effects. Front. Microbiol. 2015, 6, 147380. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, H. Enacting Partner Specificity in Legume–Rhizobia Symbioses. Abiotech 2025, 6, 311–327. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and Molecular Mechanisms Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 2018, 9, 334639. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing Nitrogen-Fixing Symbiosis with Legumes: How Many Rhizobium Recipes? Trends Microbiol. 2009, 17, 458–466. [Google Scholar] [CrossRef]
- Ovtsyna, A.O.; Geurts, R.; Bisseling, T.; Lugtenberg, B.J.J.; Tikhonovich, I.A.; Spaink, H.P. Restriction of Host Range by the Sym2 Allele of Afghan Pea Is Nonspecific for the Type of Modification at the Reducing Terminus of Nodulation Signals. Mol. Plant-Microbe Interact. 2007, 11, 418–422. [Google Scholar] [CrossRef]
- Lepetit, M.; Brouquisse, R. Control of the Rhizobium–Legume Symbiosis by the Plant Nitrogen Demand Is Tightly Integrated at the Whole Plant Level and Requires Inter-Organ Systemic Signaling. Front. Plant Sci. 2023, 14, 1114840. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bagy, M.K.; El-enany, A.W.E.S.; Bashandy, S.R. Activation of Rhizobium Tibeticum with Flavonoids Enhances Nodulation, Nitrogen Fixation, and Growth of Fenugreek (Trigonella foenum-graecum L.) Grown in Cobalt-Polluted Soil. Arch. Environ. Contam. Toxicol. 2014, 66, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, M.H.; Nafady, N.A.; Bashandy, S.R.; Hassan, A.A. Mitigation of Effect of Salt Stress on the Nodulation, Nitrogen Fixation and Growth of Chickpea (Cicer Arietinum L.) by Triple Microbial Inoculation. Rhizosphere 2019, 10, 100148. [Google Scholar] [CrossRef]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in Rhizobial Inoculant Production and Use. Plant Soil. 2001, 230, 21–30. [Google Scholar] [CrossRef]
- Watanabe, R.; Artigas Ramirez, M.D.; Agake, S.I.; Bellingrath-Kimura, S.D.; Lewandowska, S.; Onishi, Y.; Nishikawa, Y.; Takeyama, H.; Yasuda, M.; Ohkama-Ohtsu, N. Genetic Characterization and Symbiotic Performance of Soybean Rhizobia Under Cold and Water-Deficient Conditions in Poland. Plants 2025, 14, 1786. [Google Scholar] [CrossRef]
- Solano, B.R.; Jarecki, W. Soybean Response to Seed Inoculation or Coating with Bradyrhizobium japonicum and Foliar Fertilization with Molybdenum. Plants 2023, 12, 2431. [Google Scholar] [CrossRef]
- Shimoia, E.P.; Da-Silva, C.J.; Posso, D.A.; da Silva Martins, T.; Agualongo, D.A.P.; de Oliveira, A.C.B.; do Amarante, L. Co-Inoculation of Seeds with Bradyrhizobium, Azospirillum, and Rhizophagus Improves Nitrogen Assimilation and Growth in Soybean Plants Subjected to Waterlogging. Russ. J. Plant Physiol. 2023, 70, 146. [Google Scholar] [CrossRef]
- Keyser, H.H.; Li, F. Potential for Increasing Biological Nitrogen Fixation in Soybean. Biol. Nitrogen. Fixat. Sustain. Agric. 1992, 141, 119–135. [Google Scholar] [CrossRef]
- Zilli, J.É.; Pacheco, R.S.; Gianluppi, V.; Smiderle, O.J.; Urquiaga, S.; Hungria, M. Biological N2 Fixation and Yield Performance of Soybean Inoculated with Bradyrhizobium. Nutr. Cycl. Agroecosyst 2021, 119, 323–336. [Google Scholar] [CrossRef]
- Tian, C.F.; Zhou, Y.J.; Zhang, Y.M.; Li, Q.Q.; Zhang, Y.Z.; Li, D.F.; Wang, S.; Wang, J.; Gilbert, L.B.; Li, Y.R.; et al. Comparative Genomics of Rhizobia Nodulating Soybean Suggests Extensive Recruitment of Lineage-Specific Genes in Adaptations. Proc. Natl. Acad. Sci. USA 2012, 109, 8629–8634. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.F.; Ormeño-Orrillo, E.; Souza, R.C.; Rodrigues, E.P.; Almeida, L.G.P.; Barcellos, F.G.; Batista, J.S.S.; Nakatani, A.S.; Martínez-Romero, E.; Vasconcelos, A.T.R.; et al. Comparative Genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium Diazoefficiens CPAC 7: Elite Model Strains for Understanding Symbiotic Performance with Soybean. BMC Genom. 2014, 15, 420. [Google Scholar] [CrossRef]
- Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. The Success of BNF in Soybean in Brazil. Plant Soil. 2003, 252, 1–9. [Google Scholar] [CrossRef]
- Maluk, M.; Giles, M.; Wardell, G.E.; Akramin, A.T.; Ferrando-Molina, F.; Murdoch, A.; Barros, M.; Beukes, C.; Vasconçelos, M.; Harrison, E.; et al. Biological Nitrogen Fixation by Soybean (Glycine max [L.] Merr.), a Novel, High Protein Crop in Scotland, Requires Inoculation with Non-Native Bradyrhizobia. Front. Agron. 2023, 5, 1196873. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, D.; Kapoor, N.; Ohri, P. Insights into the Biodiversity, Mechanisms and Plant Growth Promoting Effects of Bradyrhizobium and Their Potential in Sustainable Agriculture. Pedosphere 2025, in press. [Google Scholar] [CrossRef]
- Banasiewicz, J.; Gumowska, A.; Hołubek, A.; Orzechowski, S. Adaptations of the Genus Bradyrhizobium to Selected Elements, Heavy Metals and Pesticides Present in the Soil Environment. Curr. Issues Mol. Biol. 2025, 47, 205. [Google Scholar] [CrossRef]
- van Rossum, D.; Muyotcha, A.; de Hoop, B.M.; van Verseveld, H.W.; Stouthamer, A.H.; Boogerd, F.C. Soil Acidity in Relation to Groundnut-Bradyrhizobium Symbiotic Performance. Plant Soil. 1994, 163, 165–175. [Google Scholar] [CrossRef]
- Marinković, J.; Miljaković, D.; Đorđević, V.; Vasiljević, M.; Tamindžić, G.; Miladinović, J.; Vasiljević, S. Perspectives of Bradyrhizobium and Bacillus Inoculation for Improvement of Soybean Tolerance to Water Deficit. Agronomy 2024, 14, 2692. [Google Scholar] [CrossRef]
- Pan, H.; Shim, A.; Lubin, M.B.; Belin, B.J. Hopanoid Lipids Promote Soybean–Bradyrhizobium Symbiosis. mBio 2024, 15, e02478-23. [Google Scholar] [CrossRef]
- Martyniuk, S. Scientific and Practical Aspects of Legumes Symbiosis with Root-Nodule Bacteria. Pol. J. Agron. 2012, 9, 17–22. (In Polish) [Google Scholar]
- Symanowicz, B.; Skorupka, W. Effect of Mineral Fertilization on Nitrogenase Activity, Yield, Nitrogen Content and Uptake with Alfalfa (Medicago sativa L.) Yield. J. Elem. 2019, 24, 181–191. [Google Scholar] [CrossRef]
- Krysciak, D.; Orbegoso, M.R.; Schmeisser, C.; Streit, W.R. Molecular Keys to Broad Host Range in Sinorhizobium fredii NGR234, USDA257, and HH103. Biol. Nitrogen. Fixat. 2015, 1–2, 325–336. [Google Scholar] [CrossRef]
- Krysciak, D.; Grote, J.; Orbegoso, M.R.; Utpatel, C.; Förstner, K.U.; Li, L.; Schmeisser, C.; Krishnan, H.B.; Streit, W.R. RNA Sequencing Analysis of the Broad-Host-Range Strain Sinorhizobium fredii NGR234 Identifies a Large Set of Genes Linked to Quorum Sensing-Dependent Regulation in the Background of a TraI and NgrI Deletion Mutant. Appl. Environ. Microbiol. 2014, 80, 5655. [Google Scholar] [CrossRef] [PubMed]
- Biondi, E.G.; Tatti, E.; Comparini, D.; Giuntini, E.; Mocali, S.; Giovannetti, L.; Bazzicalupo, M.; Mengoni, A.; Viti, C. Metabolic Capacity of Sinorhizobium (Ensifer) Meliloti Strains as Determined by Phenotype MicroArray Analysis. Appl. Environ. Microbiol. 2009, 75, 5396. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, J.; Tian, C.-F.; Liu, S.; Jiao, J.; Tian, C.-F. Adaptive Evolution of Rhizobial Symbiosis beyond Horizontal Gene Transfer: From Genome Innovation to Regulation Reconstruction. Genes. 2023, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Flores, A.; Heredia-Martínez, L.G.; Torres-Cortes, G.; Díaz-Santos, E. Harnessing Microalgae and Cyanobacteria for Sustainable Agriculture: Mechanistic Insights and Applications as Biostimulants, Biofertilizers and Biocontrol Agents. Agriculture 2025, 15, 1842. [Google Scholar] [CrossRef]
- Álvarez, C.; Jimenez-Ríos, L.; Iniesta-Pallares, M.; Jurado-Flores, A.; Molina-Heredia, F.P.; Ng, C.K.Y.; Mariscal, V. Symbiosis between Cyanobacteria and Plants: From Molecular Studies to Agronomic Applications. J. Exp. Bot. 2023, 74, 6145–6157. [Google Scholar] [CrossRef]
- Issa, A.A.; Abd-Alla, M.H.; Ohyama, T.; Issa, A.A.; Abd-Alla, M.H.; Ohyama, T. Nitrogen Fixing Cyanobacteria: Future Prospect. In Advances in Biology and Ecology of Nitrogen Fixation; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Subramanian, G.; Shanmugasundaram, S. Uninduced Ammonia Release by the Nitrogen-Fixing Cyanobacterium Anabaena. FEMS Microbiol. Lett. 1986, 37, 151–154. [Google Scholar] [CrossRef][Green Version]
- Xu, P.; Wang, E. Diversity and Regulation of Symbiotic Nitrogen Fixation in Plants. Curr. Biol. 2023, 33, R543–R559. [Google Scholar] [CrossRef]
- Rousk, K. Moss-Cyanobacteria Associations: A Model for Studying Symbiotic Interactions and Evolutionary Strategies. Am. J. Bot. 2025, 112, e70086. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Saadaoui, I.; Bibi, A.; Al-Ghouti, M.; Abu-Dieyeh, M.H. Applications, Advancements, and Challenges of Cyanobacteria-Based Biofertilizers for Sustainable Agro and Ecosystems in Arid Climates. Bioresour. Technol. Rep. 2024, 25, 101789. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biofertilizing Effect of Soil Cyanobacterium Anabaena Cylindrica–Based Formulations on Wheat Growth, Physiology, and Soil Fertility. Agriculture 2025, 15, 189. [Google Scholar] [CrossRef]
- Nawaz, T.; Fahad, S.; Gu, L.; Xu, L.; Zhou, R. Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security. Nitrogen. 2025, 6, 16. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Tazart, Z.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Impact of a Soil Cyanobacteria Consortium-Based Bioinoculant on Tomato Growth, Yield, and Fruit Quality. Plants 2025, 14, 2034. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef]
- Hamad, H.S.; Bleih, E.M.; Gewaily, E.E.; Abou Elataa, A.E.; El Sherbiny, H.A.; Abdelhameid, N.M.; Rehan, M. Cyanobacteria Application Ameliorates Floral Traits and Outcrossing Rate in Diverse Rice Cytoplasmic Male Sterile Lines. Plants 2022, 11, 3411. [Google Scholar] [CrossRef]
- Rahman, A.; Fares, A.; Veettil, A.V.; Mohtar, R.; Awal, R. A Critical Review of the Microalgae and Cyanobacteria-Based Biofertilizers: An Insight into the Cost Effectiveness of Different Algae Cultivation Strategies. Environ. Technol. Innov. 2025, 40, 104480. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Wan, Y.; Gao, B.; Rajput, V.D. Alginate-Based Composites as Novel Soil Conditioners for Sustainable Applications in Agriculture: A Critical Review. J. Environ. Manag. 2023, 348, 119133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Anju, T.; Kumar, S.; Chhapekar, S.S.; Sreedharan, S.; Singh, S.; Choi, S.R.; Ramchiary, N.; Lim, Y.P. Integrating Omics and Gene Editing Tools for Rapid Improvement of Traditional Food Plants for Diversified and Sustainable Food Security. Int. J. Mol. Sci. 2021, 22, 8093. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological Nitrogen Fixation in Non-Legume Plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Bourscheidt, M.L.B.; Gomes, F.J.; Pedreira, C.G.S.; Boote, K.J.; Hoogenboom, G.; Pereira, D.H.; Pedreira, B.C. Highlighting the Benefits of Biological Nitrogen Fixation on Agronomic, Physiological, and Nutritive Value Traits of Brachiariagrass. Eur. J. Agron. 2023, 143, 126730. [Google Scholar] [CrossRef]
- Pathak, D.; Kumar, V.; Sharma, P. Nitrate tolerance, nitrogen fixation and plant growth promotion by azospirillum—A review. Agric. Rev. 2002, 23, 31–38. [Google Scholar]
- Schultz, N.; da Silva, J.A.; Sousa, J.S.; Monteiro, R.C.; Oliveira, R.P.; Chaves, V.A.; Pereira, W.; da Silva, M.F.; Baldani, J.I.; Boddey, R.M.; et al. Inoculação de Bactérias Diazotróficas Na Cultura de Cana-de-Açúcar. Rev. Bras. Cienc. Solo 2014, 38, 407–414. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Ormeño-Orrillo, E.; López-López, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martínez-Romero, J.C.; Reddy, P.M.; Martínez-Romero, E. Nitrogen Fixation in Cereals. Front. Microbiol. 2018, 9, 1794. [Google Scholar] [CrossRef] [PubMed]
- Renoud, S.; Vacheron, J.; Abrouk, D.; Prigent-Combaret, C.; Legendre, L.; Muller, D.; Moënne-Loccoz, Y. Field Site-Specific Effects of an Azospirillum Seed Inoculant on Key Microbial Functional Groups in the Rhizosphere. Front. Microbiol. 2022, 12, 760512. [Google Scholar] [CrossRef]
- Giller, K.E.; James, E.K.; Ardley, J.; Unkovich, M.J. Science Losing Its Way: Examples from the Realm of Microbial N2-Fixation in Cereals and Other Non-Legumes. Plant Soil. 2025, 511, 1–24. [Google Scholar] [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a Free-Living Nitrogen-Fixing Bacterium Closely Associated with Grasses: Genetic, Biochemical and Ecological Aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Perini, L.; Silva, M.B.; de Sousa, N.V.; Scapim, C.A.; De Oliveira, A.L.M.; Do Amaral, A.T.; Gonçalves, L.S.A. Azospirillum Brasilense Promotes Increases in Growth and Nitrogen Use Efficiency of Maize Genotypes. PLoS ONE 2019, 14, e0215332. [Google Scholar] [CrossRef]
- Naqqash, T.; Malik, K.A.; Imran, A.; Hameed, S.; Shahid, M.; Hanif, M.K.; Majeed, A.; Iqbal, M.J.; Qaisrani, M.M.; van Elsas, J.D. Inoculation With Azospirillum spp. Acts as the Liming Source for Improving Growth and Nitrogen Use Efficiency of Potato. Front. Plant Sci. 2022, 13, 929114. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Aldrighetti, A.; Valentinuzzi, F.; Mimmo, T.; Cesco, S. Azospirillum Brasilense Inoculation Counteracts the Induction of Nitrate Uptake in Maize Plants. J. Exp. Bot. 2019, 70, 1313–1324. [Google Scholar] [CrossRef]
- Rozier, C.; Hamzaoui, J.; Lemoine, D.; Czarnes, S.; Legendre, L. Field-Based Assessment of the Mechanism of Maize Yield Enhancement by Azospirillum lipoferum CRT1. Sci. Rep. 2017, 7, 7416. [Google Scholar] [CrossRef]
- Castro-Sowinski, S.; Herschkovitz, Y.; Okon, Y.; Jurkevitch, E. Effects of Inoculation with Plant Growth-Promoting Rhizobacteria on Resident Rhizosphere Microorganisms. FEMS Microbiol. Lett. 2007, 276, 1–11. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Balsanelli, E.; Wassem, R.; Marin, A.M.; Brusamarello-Santos, L.C.C.; Schmidt, M.A.; Tadra-Sfeir, M.Z.; Pankievicz, V.C.S.; Cruz, L.M.; Chubatsu, L.S.; et al. Herbaspirillum-Plant Interactions: Microscopical, Histological and Molecular Aspects. Plant Soil. 2012, 356, 175–196. [Google Scholar] [CrossRef]
- Alves, G.C.; dos Santos, C.L.R.; Zilli, J.E.; dos Reis Junior, F.B.; Marriel, I.E.; Farley, F.A.; Boddey, R.M.; Reis, V.M. Agronomic Evaluation of Herbaspirillum seropedicae Strain ZAE94 as an Inoculant to Improve Maize Yield in Brazil. Pedosphere 2021, 31, 583–595. [Google Scholar] [CrossRef]
- Li, P.; Tian, Y.; Yang, K.; Tian, M.; Zhu, Y.; Chen, X.; Hu, R.; Qin, T.; Liu, Y.; Peng, S.; et al. Mechanism of Microbial Action of the Inoculated Nitrogen-Fixing Bacterium for Growth Promotion and Yield Enhancement in Rice (Oryza sativa L.). Adv. Biotechnol. 2024, 2, 32. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Vidal, M.S.; Baldani, J.I. Exploring ACC Deaminase-Producing Bacteria for Drought Stress Mitigation in Brachiaria. Front. Plant Sci. 2025, 16, 1607697. [Google Scholar] [CrossRef]
- Irineu, L.E.S.D.S.; Soares, C.D.P.; Soares, T.S.; de Almeida, F.A.; Almeida-Silva, F.; Gazara, R.K.; Meneses, C.H.S.G.; Canellas, L.P.; Silveira, V.; Venancio, T.M.; et al. Multiomic Approaches Reveal Hormonal Modulation and Nitrogen Uptake and Assimilation in the Initial Growth of Maize Inoculated with Herbaspirillum seropedicae. Plants 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Ehinmitan, E.; Siamalube, B.; Losenge, T.; Mamati, E.; Juma, P.; Ngumi, V. Methylobacterium spp. in Sustainable Agriculture: Strategies for Plant Stress Management and Growth Promotion. Microbe 2025, 8, 100476. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Alex, T.H.H.; Ngoh, S.T.; Prithiviraj, B.; Ji, L. Leaf-Residing Methylobacterium Species Fix Nitrogen and Promote Biomass and Seed Production in Jatropha curcas. Biotechnol. Biofuels 2015, 8, 222. [Google Scholar] [CrossRef]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A Survey of Methylobacterium Species and Strains Reveals Widespread Production and Varying Profiles of Cytokinin Phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef]
- Torres Vera, R.; Bernabé García, A.J.; Carmona Álvarez, F.J.; Martínez Ruiz, J.; Fernández Martín, F. Application and Effectiveness of Methylobacterium symbioticum as a Biological Inoculant in Maize and Strawberry Crops. Folia Microbiol. 2024, 69, 121–131. [Google Scholar] [CrossRef]
- Grossi, C.E.M.; Fantino, E.; Serral, F.; Zawoznik, M.S.; Fernandez Do Porto, D.A.; Ulloa, R.M. Methylobacterium sp. 2A Is a Plant Growth-Promoting Rhizobacteria That Has the Potential to Improve Potato Crop Yield Under Adverse Conditions. Front. Plant Sci. 2020, 11, 71. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Tariq, H.; Subramanian, S.; Geitmann, A.; Smith, D.L. Bacillus and Paenibacillus as Plant Growth-Promoting Bacteria in Soybean and Cannabis. Front. Plant Sci. 2025, 16, 1529859. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a Source of Phytohormones for Use in Agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Tarkowská, D.; Rolčík, J.; Novák, O.; Palmero, D.V.; Bejai, S.; Meijer, J. Analysis of Plant Growth-Promoting Properties of Bacillus amyloliquefaciens UCMB5113 Using Arabidopsis Thaliana as Host Plant. Planta 2017, 245, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chauhan, P.S. ACC Deaminase-Producing Rhizosphere Competent Bacillus spp. Mitigate Salt Stress and Promote Zea mays Growth by Modulating Ethylene Metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Naamala, J.; Msimbira, L.A.; Antar, M.; Subramanian, S.; Smith, D.L. Cell-Free Supernatant Obtained From a Salt Tolerant Bacillus amyloliquefaciens Strain Enhances Germination and Radicle Length Under NaCl Stressed and Optimal. Front. Sustain. Food Syst. 2022, 6, 788939. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sau, G.B.; Sinha, S.; Mukherjee, S.K. Effect of Co-Inoculation of Plant Growth-Promoting Rhizobacteria on the Growth of Amaranth Plants. Arch. Agron. Soil. Sci. 2012, 58, 1387–1397. [Google Scholar] [CrossRef]
- Singh, M.; Jha, S.; Pathak, D.; Maisnam, G. Advancing Biofertilizers: The Evolution from Single-Strain Formulations to Synthetic Microbial Communities (SynCom) for Sustainable Agriculture. Discov. Plants 2025, 2, 226. [Google Scholar] [CrossRef]
- Beltrán-Medina, J.I.; Toro-Tobón, G.; Mendoza-Labrador, J.A.; Quintero-Beyoda, A.C.; Bermudez-Cordoba, M.B.; Estrada-Bonilla, G.A. Optimizing Basil Production and Fertilizer Use Efficiency with Consortia of Plant Growth-Promoting Bacteria. Front. Plant Sci. 2025, 16, 1591969. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Ali, Q.; Ashraf, S.; Kamran, M.; Rehman, A. Development of Future Bioformulations for Sustainable Agriculture. In Microbiome in Plant Health and Disease: Challenges and Opportunities; Springer: Singapore, 2019; pp. 421–446. [Google Scholar] [CrossRef]
- Figiel, S.; Rusek, P.; Ryszko, U.; Brodowska, M.S. Microbially Enhanced Biofertilizers: Technologies, Mechanisms of Action, and Agricultural Applications. Agronomy 2025, 15, 1191. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Kannan, J.; Janaki, D. Liquid Biofertilizer-A Boon to Sustainable Agriculture. Res. Bio. Today 2020, 2, 915–918. [Google Scholar]
- Chanratana, M.; Han, G.H.; Melvin Joe, M.; Roy Choudhury, A.; Sundaram, S.; Halim, M.A.; Sa, T. Evaluation of Chitosan and Alginate Immobilized Methylobacterium Oryzae CBMB20 on Tomato Plant Growth. Arch. Agron. Soil. Sci. 2018, 64, 1489–1502. [Google Scholar] [CrossRef]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and Perspectives of the Use of Alginate as a Polymer Matrix for Microorganisms Applied in Agro-Industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Alenezi, F.N.; Belbahri, L. Recent Advances in Encapsulation Techniques of Plant Growth-Promoting Microorganisms and Their Prospects in the Sustainable Agriculture. Appl. Sci. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The Potential of Biochar as a Microbial Carrier for Agricultural and Environmental Applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Francés, J.; Montesinos, E.; Bonaterra, A. Improvement of a Dry Formulation of Pseudomonas fluorescens EPS62e for Fire Blight Disease Biocontrol by Combination of Culture Osmoadaptation with a Freeze-Drying Lyoprotectant. J. Appl. Microbiol. 2014, 117, 1122–1131. [Google Scholar] [CrossRef]
- Stephan, D.; Da Silva, A.P.M.; Bisutti, I.L. Optimization of a Freeze-Drying Process for the Biocontrol Agent Pseudomonas spp. and Its Influence on Viability, Storability and Efficacy. Biol. Control 2016, 94, 74–81. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Liu, J.; Shen, Z.; Liu, Z.; Gu, H.; Hu, X.; Yu, Z.; Li, Y.; Jin, J.; et al. Biofertilizers Enhance Soil Fertility and Crop Yields Through Microbial Community Modulation. Agronomy 2025, 15, 1572. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Penton, C.R.; Xiong, W.; Liu, C.; Wang, R.; Liu, Z.; Xu, X.; Li, R.; Shen, Q. Reshaping the Rhizosphere Microbiome by Bio-Organic Amendment to Enhance Crop Yield in a Maize-Cabbage Rotation System. Appl. Soil. Ecol. 2019, 142, 136–146. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial Biofilms in Nature: Unlocking Their Potential for Agricultural Applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef]
- Li, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Biofilms Formation in Plant Growth-Promoting Bacteria for Alleviating Agro-Environmental Stress. Sci. Total Environ. 2024, 907, 167774. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Zhao, M.; Yuan, J.; Kowalchuk, G.A.; Shen, Q. Root Exudates Mediate Plant Defense against Foliar Pathogens by Recruiting Beneficial Microbes. Soil Ecol. Lett. 2021, 3, 42–51. [Google Scholar] [CrossRef]
- Verma, S.K.; Dave, A.; Israr, J.; Singh, D.; Misra, S. Rhizosphere Engineering for Agriculture and Ecological Sustainability. In Plant-Microbiome Interactions for Climate-Resilient Agriculture; Springer: Singapore, 2025; pp. 259–279. [Google Scholar] [CrossRef]
- Russell, S.J.; Garcia, A.K.; Kaçar, B. A CRISPR Interference System for Engineering Biological Nitrogen Fixation. mSystems 2024, 9, e00155-24. [Google Scholar] [CrossRef] [PubMed]
- Diwan, D.; Rashid, M.M.; Vaishnav, A. Current Understanding of Plant-Microbe Interaction through the Lenses of Multi-Omics Approaches and Their Benefits in Sustainable Agriculture. Microbiol. Res. 2022, 265, 127180. [Google Scholar] [CrossRef]
- Venkataraman, M.; Yñigez-Gutierrez, A.; Infante, V.; MacIntyre, A.; Fernandes-Júnior, P.I.; Ané, J.M.; Pfleger, B. Synthetic Biology Toolbox for Nitrogen-Fixing Soil Microbes. ACS Synth. Biol. 2023, 12, 3623–3634. [Google Scholar] [CrossRef]
- Telles, T.S.; Nogueira, M.A.; Hungria, M. Economic Value of Biological Nitrogen Fixation in Soybean Crops in Brazil. Environ. Technol. Innov. 2023, 31, 103158. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Parra Cota, F.I.; Cira Chávez, L.A.; García Ortega, L.F.; Estrada Alvarado, M.I.; Santoyo, G.; de los Santos-Villalobos, S. Microbial Inoculants in Sustainable Agriculture: Advancements, Challenges, and Future Directions. Plants 2025, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Arrobas, M.; Correia, C.M.; Rodrigues, M.Â. Methylobacterium symbioticum Applied as a Foliar Inoculant Was Little Effective in Enhancing Nitrogen Fixation and Lettuce Dry Matter Yield. Sustainability 2024, 16, 4512. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]

| Mechanism | Key Processes | Microbial Agents/Examples | Main Effects on Nitrogen Use Efficiency (NUE) | Reference |
|---|---|---|---|---|
| Nutrient Mobilization | Solubilization of P, K, and micronutrients through secretion of organic acids, phosphatases, and siderophores | Bacillus subtilis, Azospirillum brasilense | Increased availability of co-limiting nutrients (P, K), enhanced nitrate reductase activity, improved nitrogen assimilation | [35,36,39] |
| Nutrient-cycling regulation | Suppression of nitrification and denitrification; enhancement of Biological Nitrification Inhibition (BNI) and N retention as NH4+ | Nitrosomonas inhibition by Brachiaria humidicola, Sorghum spp.; microbial consortia promoting anammox or partial nitrification–denitrification | Reduced N2O emissions, minimized nitrate leaching, improved synchronization of N availability with plant demand | [40,41] |
| Root Growth Stimulation | Hormonal modulation (auxin, cytokinin, gibberellin), enhanced root hair formation, mycorrhizal associations | Azospirillum brasilense, Bacillus spp., arbuscular mycorrhizal fungi (AMF) | Greater root surface area and soil exploration, improved N absorption and assimilation, enhanced plant vigor | [42,43,44] |
| Stress Mitigation | Activation of antioxidant enzymes, osmolyte accumulation, photosynthetic stabilization, stress-responsive gene expression | Bacillus spp., Pseudomonas spp., AMF | Maintained N metabolism under salinity/drought, improved nitrate reductase and glutamine synthetase activity | [45,46] |
| Genus | Representative Species | Principal Host | Ecological Distribution | Agricultural Relevance | References |
|---|---|---|---|---|---|
| Rhizobium | R. leguminosarum bv. viciae R. leguminosarum bv. phaseoli R. leguminosarum bv. trifolii R. leguminosarum bv. etli | Vetch, peas, lentils Common bean Clover Beans, common bean | Temperate, metal soils Sensitive to acidity, drought Pasture-adapted, cool climates Prevalent in Latin America | European grain legumes Requires inoculation in many regions Important in forage systems Widespread use in smallholder systems | [63,64,65,66] |
| Bradyrhizobium | B. japonicum B. diazoefficiens B. elkanii B. yuanmingense B. liaoningense B. canariense | Soybean Soybean Common bean, soybean Lespedeza spp., soybean Soybean, wild legumes Lupinus, Genista | Acid-tolerant; tropical/subtropical Efficient, microaerobic adaptation Heat- and drought-tolerant Acid-soil adaptation Cold-adapted; found in China Mediterranean legumes | Major soybean inoculant worldwide Widely used in commercial inoculants Valuable in tropical regions Relevant for subtropical forage legumes Soybean cultivation in cooler climates Reforestation and marginal lands | [67,68,69] |
| Sinorhizobium | S. meliloti S. fredii | Melilot, alfalfa, fenugreek Soybean, bean, legumes | Alkaline soils; drought tolerance Broad host range; stress-adaptable | Model for molecular symbiosis Nodulates > 200 legumes; Asia | [70,71] |
| Mesorhizobium | M. loti M. huakuii | Lotus, lupin Chinese milk vetch | Acid soils; low fertility East Asia distribution | Model with Lotus japonicus Manure crop in rice systems | [72,73] |
| Cyanobacteria | Nostoc punctiforme Anabaena azollae (N. azollae) Nostoc commune Nostoc gunnerae (symbiont) | Hornworts, liverworts, Aquatic fern Azolla Free-living associations Gunnera (angiosperm) | Cosmopolitan; oligotrophic soils Aquatic habitats, flooded rice Tolerance to desiccation and UV Tropical wet environments Subtropical to tropical soils | Nitrogen input in pioneer ecosystems Rice systems Nitrogen input in arid ecosystems Ecological model for co-evolution Cycad survival in oligotrophic soils | [74,75,76,77] |
| Azospirillum | A. brasilense | Maize, wheat, rice, sorghum | Rhizosphere colonizer; drought and nutrient stress tolerant | Commercial inoculant | [78] |
| Herbaspirillum | H. seropedicae | Sugarcane, maize, rice | Endophytic; tropical soils | Improves N uptake in grasses; tested as inoculant for sugarcane | [79] |
| Methylobacterium | M. oryzae | Rice, wheat | Leaf and root colonizer; utilizes methanol; moderate stress adaptation | Enhances germination, growth, and yield in cereals | [80] |
| Bacillus | B. subtilis | Maize, soybean, vegetables | Endospore-forming; drought, salinity, and heat tolerant | Biofertilizer/biocontrol agent; improves nutrient uptake and stress resilience | [81] |
| Name | Microorganism | Concentra. | Crop (s) | Company | Reference |
|---|---|---|---|---|---|
| Pivot Bio PROVEN®40 OS | Kosakonia sacchari 6-5687 (Ks6-5687)/Klebsiella variicola 137-2253 (Kv137-2253) | ≥1 × 109 CFU g−1 | Maize (Corn) | Pivot Bio Inc. (Berkeley, CA, USA) | https://www.pivotbio.com/product-proven40-corn (accessed on 27 October 2025) |
| Pivot Bio RETURN® | Klebsiella variicola 137-1036 (Kv137-1036) | 4 × 108 CFU g−1 | Wheat | Pivot Bio Inc. (Berkeley, CA, USA) | https://www.pivotbio.com/product-return-wheat (accessed on 27 October 2025) |
| Pivot Bio CERT-N™ | Kosakonia sacchari 6-5687 (Ks6-5687)/Klebsiella variicola 137-2253 (Kv137-2253) | ≥1 × 109 CFU g−1 | Cotton | Pivot Bio Inc. (Berkeley, CA, USA) | https://www.pivotbio.com/product-cert-n-cotton (accessed on 27 October 2025) |
| Always-N™/FixiN 33 | Proprietary microbial consortium (N-fixing) | Not declare | Row Crops | BioConsortia Inc. (Davis, CA, USA) | https://bioconsortia.com/bioconsortia-expands-nitrogen-fixing-microbial-products-for-row-crops/ (accessed on 27 October 2025) |
| BlueN® /Utrisha®N | Methylobacterium symbioticum (SB23) | 3 × 107 CFU g−1 | Row crops, Horticultural, Olives and Grapes | Corteva Agriscience (Indianapolis, IN, USA) | https://www.corteva.co.uk/products-and-solutions/biologicals/bluen.html (accessed on 27 October 2025) |
| Nutri-Life Bio-N™ | Azotobacter vinelandii | Not declared | Broadacre & horticultural crops | Nutri-Tech Solutions (Yandina, Queenslan, Australia) | https://nutri-tech.com.au/products/bio-n (accessed on 27 October 2025) |
| Life Force Bio-N™ | Azotobacter chroococcum | 1 × 108 CFU mL−1 | Broadacre & horticultural crops | Nutri-Tech Solutions (Yandina, Queenslan, Australia) | https://regenagsolutions.ca/biologicals/life-force-bio-n/ (accessed on 27 October 2025) |
| AZOFIX® | Azotobacter vinelandii MVY-010 | 1 × 109 CFU mL−1 | Grassland, arable, Fruit and vegetables | UAB Bioenergy LT (Panevezys, Lithuania) | https://vatzum.lrv.lt/media/viesa/saugykla/2024/2/xMNuAnrcjXA.pdf (accessed on 27 October 2025) |
| Azofix Plus | Paenibacillus polymyxa MVY-024 | 1.2 × 109 CFU mL−1 | Grassland, arable, Fruit and vegetables | UAB Bioenergy LT (Panevezys, Lithuania) | https://www.bioenergy.lt/en/product/azofix-plus/ (accessed on 27 October 2025) |
| N-FOLIAR | Methylobacterium phyllosphaerae MVY-033 | 1.2 × 109 CFU mL−1 | Grassland, arable, Fruit and vegetables | UAB Bioenergy LT (Panevezys, Lithuania) | https://vatzum.lrv.lt/media/viesa/saugykla/2024/2/xMNuAnrcjXA.pdf (accessed on 27 October 2025) |
| Nientris® | Azotobacter salinestris strain CECT 9690 Wickerhamomyces anomalus strain CECT 13172 | 1 × 107 CFU g−1 1 × 107 CFU g−1 | Grassland, arable, Fruit and vegetables | Syngenta Crop Protection AG (Basel Switzerland) | https://www.syngenta.co.uk/product/crop-protection/biostimulant/nientris (accessed on 27 October 2025) |
| Accolade® | Azospirillum brasilense | 1 × 109 CFU g−1 | Row Crops | Verdesian (Cary, NC, USA) | https://vlsci.com/products/accolade/ (accessed on 27 October 2025) |
| NEOFORCE N FIXER | Azospirillum brasilense Azotobacter vinelandii Rhizobium leguminosarum | 1 × 108 CFU mL−1 1 × 108 CFU mL−1 1 × 108 CFU mL−1 | Grassland, arable, Fruit and vegetables | Fertiberia S.A (Madrid, Spain) | https://www.fertiberiatech.com/ (accessed on 27 October 2025) |
| N-Leaf | Methylobacterium brachiatum AGN12 Methylobacterium pseudosasicola AGN13 Arthrobacter globiformis AGN14 | 1 × 109 CFU g−1 1 × 109 CFU g−1 1 × 109 CFU g−1 | Grassland, arable, Fruit and vegetables | De Sangosse (Pont-du-Casse, France) | https://desangosse.com.au/produit/n-leaf/ (accessed on 27 October 2025) |
| Simbiotic ® | Bacillus megaterium CECT 30994 | 2 × 108 CFU g−1 | Grassland, arable, Fruit and vegetables | CERES Biotics Tech S.L (San Fernando de Henares, Madrid, Spain) | https://ceresbiotics.com/en/products/simbiotic/ (accessed on 27 October 2025) |
| Symbius | Bacillus altitudinis strain NTC/BC/01 | 4.853 × 1010 CFU mL−1 | Horticultural and woody crops | Nostoc Biotech (Beer Sheva, Israel) | https://nostocbiotech.group/ (accessed on 27 October 2025) |
| Amylis | Bacillus amyloliquefaciens cepa I4995 Bacillus amyloliquefaciens strainI4996 | 5 × 108 CFU mL−1 5 × 108 CFU mL−1 | Horticultural and woody crops | De Sangosse De Sangosse (Pont-du-Casse, France) | https://www.desangosse.co.nz/produit/amylis/ (accessed on 27 October 2025) |
| Cell-Tech® XC | Bradyrhizobium japonicum | 1 × 1010 CFU mL−1 | Soybean | Novonesis Plant Biosolutions A/S (Kongens Lyngby, Denmark) | https://www.novonesis.com/en/biosolutions/animal-and-plant/plant/soybeans/cell-tech-xc (accessed on 27 October 2025) |
| LALFIX® LIQUID Pea & Lentil | Rhizobium leguminosarum biovar viciae | 8 × 108 CFU mL−1 | Pea, lentil, and faba bean | Lallemand Plant Care (Montreal, Canada) | https://www.lallemandplantcare.com/en/usa/products/lalfix-liquid-pea-lentil/ (accessed on 27 October 2025) |
| Nodulator® PRO/IP Plus | Bradyrhizobium japonicum (strain 532C) | 1 × 1010 CFU/ml | Soybean | BASF (Limburgerhof, Germany) | https://agriculture.basf.ca/east/en/products/solutions/nodulator-ip-plus.html (accessed on 27 October 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero, J.; Ramírez-Santos, A.; Díaz-Santos, E.; Torres-Cortés, G. Biofertilizers for Enhanced Nitrogen Use Efficiency: Mechanisms, Innovations, and Challenges. Nitrogen 2025, 6, 111. https://doi.org/10.3390/nitrogen6040111
Herrero J, Ramírez-Santos A, Díaz-Santos E, Torres-Cortés G. Biofertilizers for Enhanced Nitrogen Use Efficiency: Mechanisms, Innovations, and Challenges. Nitrogen. 2025; 6(4):111. https://doi.org/10.3390/nitrogen6040111
Chicago/Turabian StyleHerrero, Joaquín, Adrián Ramírez-Santos, Encarnación Díaz-Santos, and Gloria Torres-Cortés. 2025. "Biofertilizers for Enhanced Nitrogen Use Efficiency: Mechanisms, Innovations, and Challenges" Nitrogen 6, no. 4: 111. https://doi.org/10.3390/nitrogen6040111
APA StyleHerrero, J., Ramírez-Santos, A., Díaz-Santos, E., & Torres-Cortés, G. (2025). Biofertilizers for Enhanced Nitrogen Use Efficiency: Mechanisms, Innovations, and Challenges. Nitrogen, 6(4), 111. https://doi.org/10.3390/nitrogen6040111







