Permeable Organic Barriers as Effective Tools for Reducing Emissions of Nitrogen Compounds and PCBs from Manure to Groundwater
Abstract
1. Introduction
2. Materials and Methods
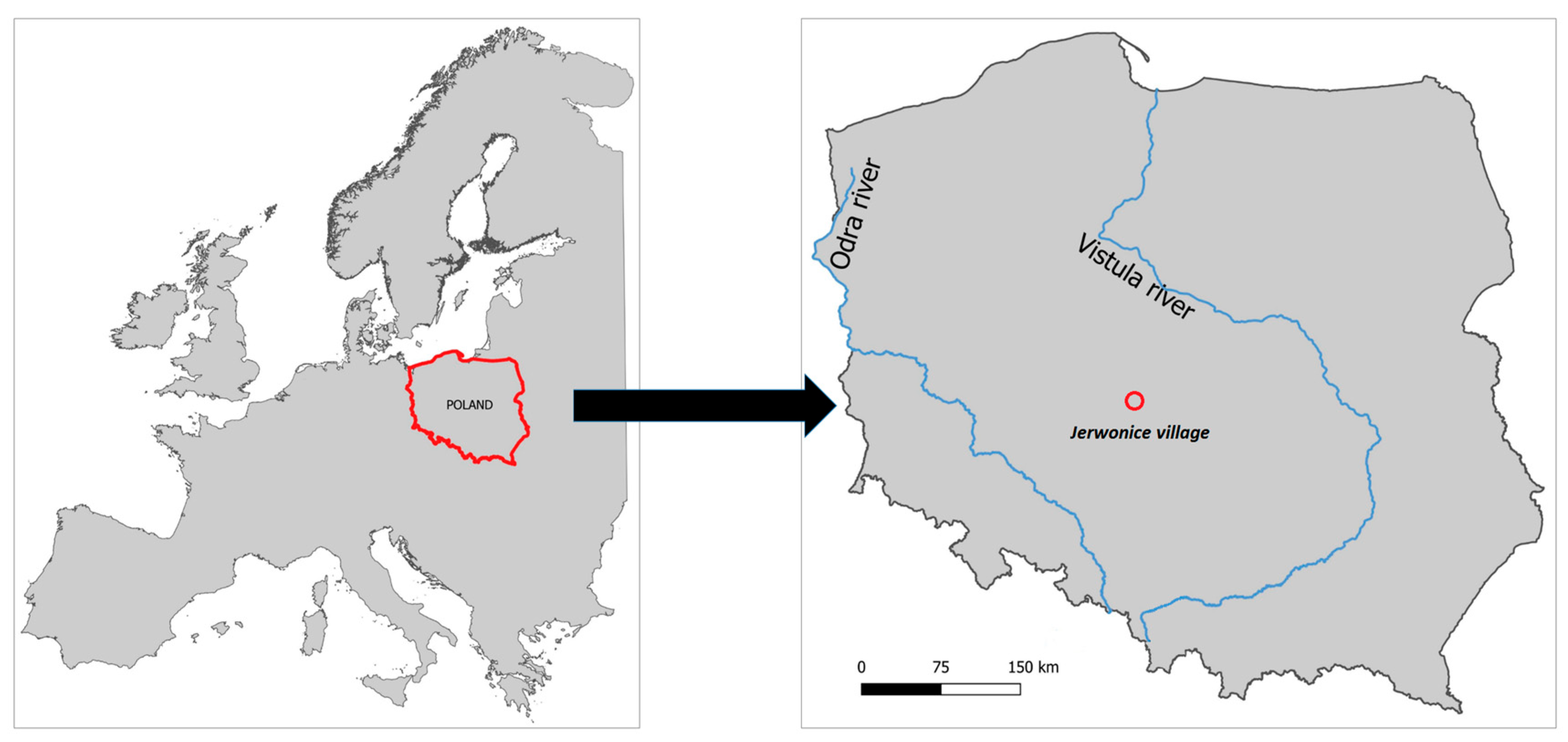
2.1. Study Site and Construction of the Permeable Organic Barrier
2.2. Measurements of Physical Parameters
2.3. Chemical Analysis
2.3.1. Nitrogen Compounds
2.3.2. PCBs
2.4. Molecular Analysis
2.4.1. Denitrifying Bacteria
2.4.2. PCB-Degrading Bacteria
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Parameters of Groundwater in Permeable Organic Barrier
3.2. Chemical Parameters of Groundwater in Permeable Organic Barrier
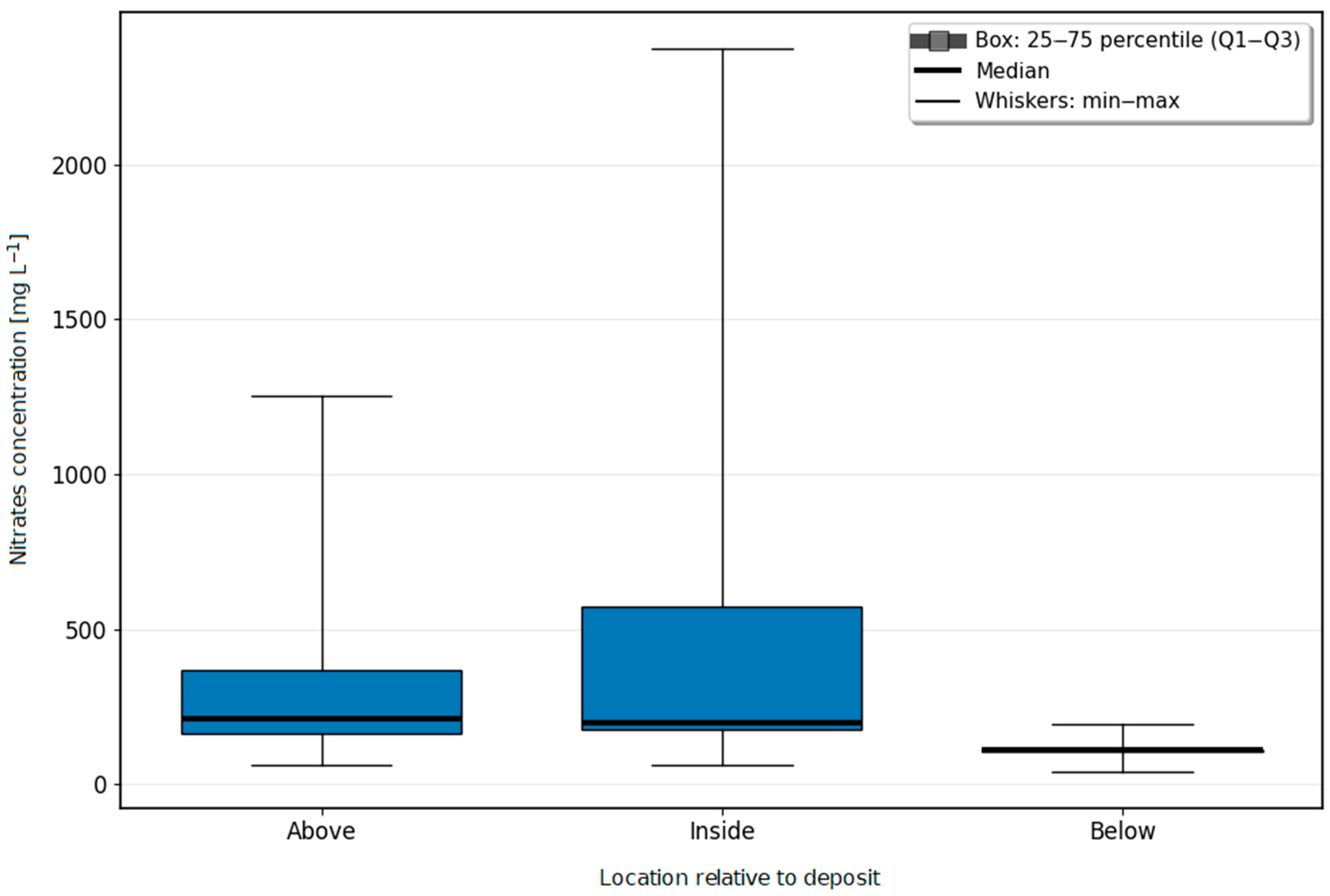
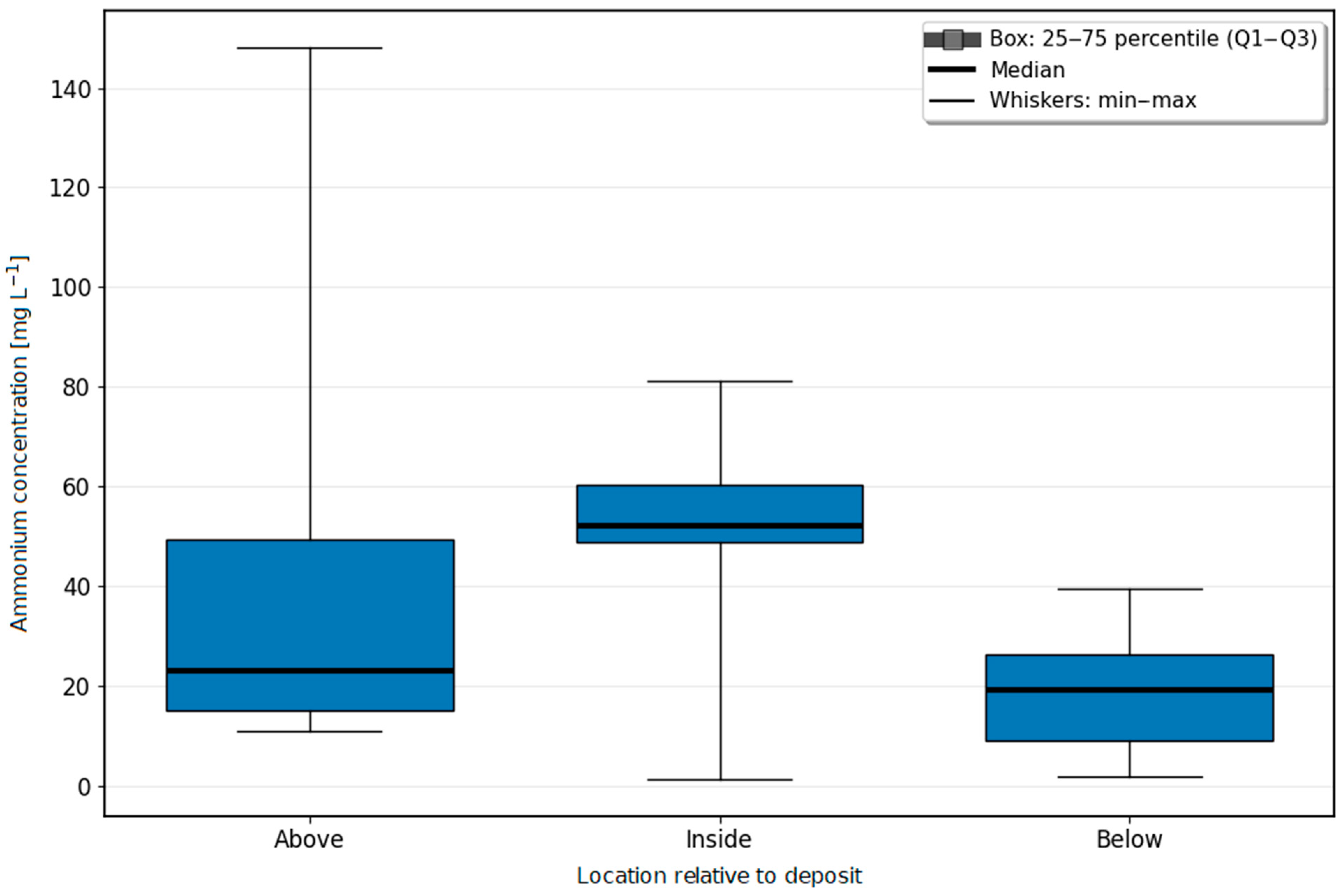
Changes in the Concentrations of Nitrogen Compounds
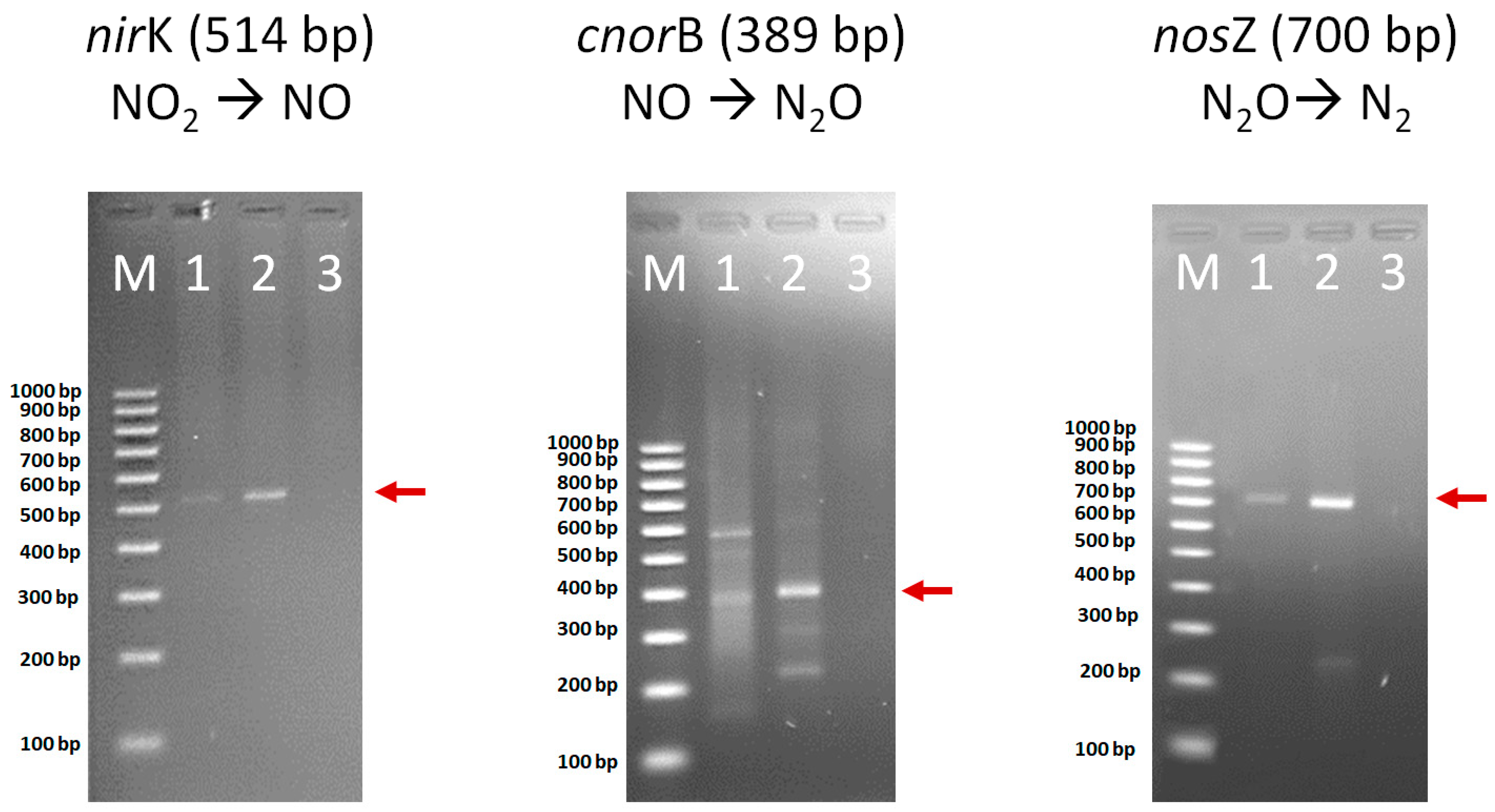
3.3. Bacterial Transformation of Nitrite to Gaseous Nitrogen
3.4. PCBs in the Groundwater of the Permeable Organic Barrier
3.4.1. Changes in PCB Concentrations
3.4.2. Bacterial Degradation of PCBs
3.5. Seasonal Influence on the Operation of the Permeable Organic Barrier
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations—Department of Economic and Social Affairs. World Population Prospects 2019. Report ST/ESA/SER.A/423; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Report Greenpeace. Feeding the Problem—The Dangerous Intensification of Animal Farming in Europe; Greenpeace European Unit: Brussels, Belgium, 2019; Available online: https://www.greenpeace.org/static/planet4-eu-unit-stateless/2019/02/83254ee1-190212-feeding-the-problem-dangerous-intensification-of-animal-farming-in-europe.pdf (accessed on 3 October 2025).
- Kupiec, J.M. Wieloaspektowa Ocena Wywieranej Presji Gospodarstw Rolnych na Środowisko [Multi-aspect Assessment of the Pressure of Farms on the Environment]; Poznań University of Life Sciences Publishing House: Poznań, Poland, 2023; p. 169. [Google Scholar] [CrossRef]
- Kyllmann, C.; Clean Energy Wire. German Meat Consumption Falls To Record Low in 2023. 2024. Available online: https://www.cleanenergywire.org/news/german-meat-consumption-falls-record-low-2023 (accessed on 28 October 2025).
- Eurostat. Statistics Explained. Agricultural Production—Livestock and Meat. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_livestock_and_meat (accessed on 28 October 2025).
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023; Available online: https://chooser.crossref.org/?doi=10.1787%2F08801ab7-en (accessed on 28 October 2025).
- Fernández, E.; Grilli, A.; Alvarez, D.; Aravena, R. Evaluation of nitrate levels in groundwater under agricultural fields in two pilot areas in central Chile: A hydrogeological and geochemical approach. Hydrol. Processes. 2017, 31, 1206–1224. [Google Scholar] [CrossRef]
- Pietrzak, S.; Nawalny, P. Wpływ długo- i krótkotrwałego składowania obornika na gruncie na zanieczyszczenie gleby i wody związkami azotu [Effect of long- and short-term storage of manure on the soil on soil and water contamination with nitrogen compounds]. Woda Śr. Obsz. Wiej. 2008, 8, 117–126. [Google Scholar]
- Fotyma, M.; Igras, J.; Kopinski, J. Nitrogen utilization and diffuse losses in agricultural crop production Temporal. In Temporal and Spatial Differences in Emission of Nitrogen and Phosphorus from Polish Territory to the Baltic Sea; Pastuszak, M., Igras, J., Eds.; National Marine Fisheries Research Institute: Gdynia, Poland; Institute of Soil Science and Plant Cultivation—State Research Institute: Puławy, Poland; Fertilizer Research Institute: Pulawy, Poland, 2012. [Google Scholar]
- Bednarek, A.; Stolarska, M.; Ubraniak, M.; Zalewski, M. Application of permeable reactive barrier for removal of nitrogen load in the agricultural areas—Preliminary results. Ecohydrol. Hydrobiol. 2010, 10, 355–362. [Google Scholar] [CrossRef]
- Stevens, J.L.; Jones, K. Quantification of PCDD/F concentrations in animal manure and comparison to the effects of the application of cattle manure and sewage sludge to agricultural land on human exposure to PCDD/Fs. Chemosphere 2003, 50, 1183–1191. [Google Scholar] [CrossRef]
- Kakimoto, H.; Oka, H.; Miyata, Y.; Yonezawa, Y.; Niikawa, A.; Kyudo, H.; Tang, N.; Toriba, A.; Kizu, R.; Hayakawa, K. Homologue and isomer distribution of dioxins observed in water samples collected from Kahokugata Lagoon and inflowing rivers. Jpn. Water Res. 2006, 40, 1929–1940. [Google Scholar] [CrossRef]
- Minomo, K.; Ohtsuka, N.; Hosono, S.H.; Nojiri, K.; Kawamura, K. Seasonal change of PCDDs/PCDFs/Dl-PCBs in the water of Ayse River, Japan: Pollution sources and their contributions to TEQ. Chemosphere 2011, 85, 188–194. [Google Scholar] [CrossRef]
- Dumortier, P.; Elskens, M.; Focant, J.F.; Goeyens, J.; Vandermeiren, K.; Pussemier, L. Potential impact of fertilization practices on human dietary intake of dioxins in Belgium. Sci. Total Environ. 2012, 423, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Elsknes, M.; Pussemier, L.; Dumortier, P.; Van Langenhove, K.; Scholl, G.; Goeyens, L.; Focant, J.F. Dioxins levels in fertilizers from Belgium: Determination and evaluation of the potential impact on soil contamination. Sci. Total Environ. 2013, 454–455, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, A.; Szklarek, S.; Zalewski, M. Nitrogen pollution removal from areas of intensive farming–comparison of various denitrification biotechnologies. Ecohydrol. Hydrobiol. 2014, 14, 132–141. [Google Scholar] [CrossRef]
- Mankiewicz-Boczek, J.; Bednarek, A.; Gągała-Borowska, I.; Serwecińska, L.; Zaborowski, A.; Kolate, E.; Pawełczyk, J.; Żaczek, A.; Dziadek, J.; Zalewski, M. The removal of nitrogen compounds from farming wastewater—The effect of different carbon substrates and different microbial activators. Ecol. Eng. 2017, 105, 341–354. [Google Scholar] [CrossRef]
- Zalewski, M. Ecohydrology and Hydrologic Engineering: Regulation of Hydrology-Biota Interactions for Sustainability. J. Hydrol. Eng. 2015, 20, A4014012. [Google Scholar] [CrossRef]
- Zalewski, M.; Kiedrzynska, E.; Wagner, I.; Izydorczyk, K.; Boczek, J.M.; Jurczak, T.; Krauze, K.; Frankiewicz, P.; Godlewska, M.; Wojtal-Frankiewicz, A.; et al. Ecohydrology and adaptation to global change. Ecohydrol. Hydrobiol. 2021, 21, 393–410. [Google Scholar] [CrossRef]
- Robertson, W.D.; Blowes, D.W.; Ptacek, C.J.; Cherry, J.A. Long-Term performance of in situ reactive barriers for nitrate remediation. Ground Water 2000, 38, 689–695. [Google Scholar] [CrossRef]
- Schipper, L.A.; Vojvodić-Vuković, M. Nitrate removal from groundwater and denitrification rates in a porous treatment wall amended with sawdust. Ecol. Eng. 2000, 14, 269–278. [Google Scholar] [CrossRef]
- Schipper, L.A.; Vojvodić-Vuković, M. Five years of nitrate removal, denitrification and carbon dynamics in a denitrification wall. Water Res. 2001, 35, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.A.; Barkle, G.F.; Hadfield, J.C.; Vojvodic-Vukovic, M.; Burgess, C.P. Hydraulic constraints on the performance of a groundwater denitrification wall for nitrate removal from shallow groundwater. J. Contam. Hydrol. 2003, 69, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.A.; Barkle, G.F.; Vojvodic-Vukovic, M. Maximum Rates of Nitrate Removal in a Denitrification Wall. J. Environ. Qual. 2005, 34, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, K.; Michalska-Hejduk, D.; Frątczak, W.; Bednarek, A.; Łapińska, M.; Jarosiewicz, P.; Kosińska, A.; Zalewski, M. Strefy Buforowe i Biotechnologie Ekohydrologiczne w Ograniczaniu Zanieczyszczeń Obszarowych [Buffer Zones and Ecohydrological Biotechnologies in Reducing Diffuse Pollution]; ERCE PAN: Łódź, Poland, 2015; ISBN 978-83-928245-1-0. [Google Scholar]
- Wibig, J.; Radziun, W. Opady atmosferyczne w województwie łódzkim w latach 1961–2015 [Precipitation in the Łódź Voivodeship in the years 1961–2015]. Acta Geogr. Lodziensia. 2019, 109, 29–47. [Google Scholar]
- Wyrwicka, A.; Steffani, S.; Urbaniak, M. The effect of PCB-contaminated sewage sludge and sediment on metabolism of cucumber plants (Cucumis sativus L.). Ecohydrol. Hydrobiol. 2014, 14, 75–82. [Google Scholar] [CrossRef]
- Urbaniak, M.; Gągała, I.; Szewczyk, M.; Bednarek, A. Leaching of PCBs and Nutrients from Soil Fertilized with Municipal Sewage Sludge. Bull. Environ. Contam. Toxicol. 2016, 97, 249–254. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.P. Development of PCR primer systems for amplification of nitrite reductase gene (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef] [PubMed]
- Braker, G.; Tiedje, J.M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef]
- Rich, J.J.; Heichen, R.S.; Bottomley, P.J.; Cromack, K.; Myrold, D.D. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl. Environ. Microbiol. 2003, 69, 5974–5982. [Google Scholar] [CrossRef] [PubMed]
- Ryslava, E.; Macek, T.; Mackova, M.; Krejcik, Z. Detection of polychlorinated biphenyldegrading bacteria in soil. In Environmental Biotechnology; Verstraete, W., Ed.; Taylor & Francis Group: London, UK, 2004; pp. 839–842. [Google Scholar]
- Uhlik, O.; Jecna, K.; Mackova, M.; Vlcek, C.; Hroudova, M.; Demnerova, K.; Paces, V.; Macek, T. Biphenyl-metabolizing bacteria in the rhizosphere of horseradish and bulk soil contaminated by polychlorinated biphenyls as revealed by stable isotope probing. Appl. Environ. Microbiol. 2009, 75, 6471–6477. [Google Scholar] [CrossRef]
- National Library of Medicine. National Center of Biotechnology Information. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 2 October 2025).
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Janžekovič, F.; Novak, T. PCA—A powerful method for analyze ecological niches. In Principal Component Analysis—Multidisciplinary Applications; Sanguansat, P., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electronica. 2001, 4, 9. [Google Scholar]
- Tomaszek, J. Biochemical transformation of nitrogen compounds in the bottom sediments of the superficial waters. Rzesz. Tech. Univ. J. 1991, 13, 1–155. [Google Scholar]
- Janosz-Rajczyk, M. Biologiczne Metody Usuwania Azotu z Wybranych wód Opadowych [Biological Methods of Nitrogen Removal from Selected Rainwater]; Wydawnictwo Politechniki Częstochowskiej: Częstochowa, Poland, 2004. [Google Scholar]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef] [PubMed]
- Tomaszek, J.A.; Czerwieniec, E. Denitrification and oxygen consumption in bottom sediments: Factors influencing rates of the processes. Hydrobiologia 2003, 504, 59–65. [Google Scholar] [CrossRef]
- Council Directive 91/676/EEC Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (the Nitrates Directive). 1991. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2002:0407:FIN:EN:PDF (accessed on 2 October 2025).
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater Against Pollution and Deterioration (the Groundwater Directive). 2016. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:372:0019:0031:EN:PDF (accessed on 2 October 2025).
- Miyahara, M.; Kim, S.W.; Zhou, S.; Fushinobu, S.; Yamada, T.; Ikeda-Ohtsubo, W.; Watanabe, A.; Miyauchi, K.; Endo, G.; Wakagi, T.; et al. Survival of the Aerobic Denitrifier Pseudomonas stutzeri Strain TR2 during Co-Culture with Activated Sludge under Denitrifying Conditions. Biosci. Biotechnol. Biochem. 2012, 76, 495–500. [Google Scholar] [CrossRef]
- Chakraborty, R.; Woo, H.; Dehal, P.; Walker, R.; Zemla, M.; Auer, M.; Goodwin, L.A.; Kazakov, A.; Novichkov, P.; Arkin, A.P.; et al. Complete genome sequence of Pseudomonas stutzeri strain RCH2 isolated from a Hexavalent Chromium [Cr(VI)] contaminated site. Stand Genomic Sci. 2017, 12, 23. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Y.; Tao, X.-Q.; Zhang, K.; Shen, D.-S.; Long, Y.-Y. Dechlorination of polychlorinated biphenyl-contaminated soil via anaerobic composting with pig manure. J. Hazard. Mater. 2013, 261, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Borja, J.; Taleon, D.M.; Auresenia, J.; Gallardo, S. Polychlorinated biphenyls and their biodegradation. Process Biochem. 2005, 40, 1999–2013. [Google Scholar] [CrossRef]
- Matturo, B.; Mascolo, G.; Rosetti, S. Microbiome changes and oxidative capability of an anaerobic PCB dechlorinating enrichment culture after oxygen exposure. New Biotechnol. 2020, 56, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, M. Biodegradation of PCDDs/PCDFs and PCBs. In Biodegradation: Engineering and Technology; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: Rijeka, Croatia, 2013; pp. 73–100. [Google Scholar] [CrossRef]
- Chakraborty, J.; Das, S. Molecular perspectives and recent advances in microbial remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2016, 23, 16883–16903. [Google Scholar] [CrossRef] [PubMed]
- Font-Nájera, A.; Serwecinska, L.; Szklarek, S.; Mankiewicz-Boczek, J. Seasonal and spatial changes of N-transforming microbial communities in sequential sedimentation-biofiltration systems—Influence of system design and environmental conditions. Int. Biodeterior. Biodegrad. 2021, 159, 105203. [Google Scholar] [CrossRef]
- Journal of Laws, No. 147, pos. 1033. ACT About Fertilizers and from Day 10 July 2007 Number 147, poz. 1033 [Ustawa o Nawozach i Nawożeniu z Dnia 10 Lipca 2007 r. (Dz.U. Nr 147, poz. 1033)]. 2007. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20071471033 (accessed on 2 October 2025).
- Pietrzak, S.; Nawalny, P. Ocena stanu jakości wód będących pod wpływem obornika posadowionego bezpośrednio na gruncie [w:] Badania chemiczne w służbie rolnictwa i ochrony środowiska [Assessment of the quality status of water under the influence of manure planted directly on the ground]. In Chemical Research in the Service of Agriculture and Environmental Protection; Sapek, B., Ed.; IMUZ: Raszyn, Poland, 2009. [Google Scholar]
- Højberg, A.L.; Hansen, A.L.; Wachniew, P.; Żurek, A.J.; Virtanen, S.; Arustine, J.; Strömqvist, J.; Rankinen, K.; Refsgaard, J.C. Review and assessment of nitrate reduction in the groundwater in the Baltic Sea Basin. J. Hydrol. Reg. Stud. 2017, 12, 50–68. [Google Scholar] [CrossRef]
- Räike, A.; Oblomkova, N.; Svendsen, L.M.; Kaspersson, R.; Haapaniemi, J.; Eklund, K.; Brynska, W.; Hytteborn, J.; Tornbjerg, H.; Kotilainen, P.; et al. Background Information on the Baltic Sea Catchment Area for the Sixth Baltic Sea Pollution Load compilation (PLC-6); HELCOM: Helsinki, Finland, 2019; Available online: https://helcom.fi/wp-content/uploads/2020/01/PLC-6-background-report.pdf (accessed on 2 October 2025).


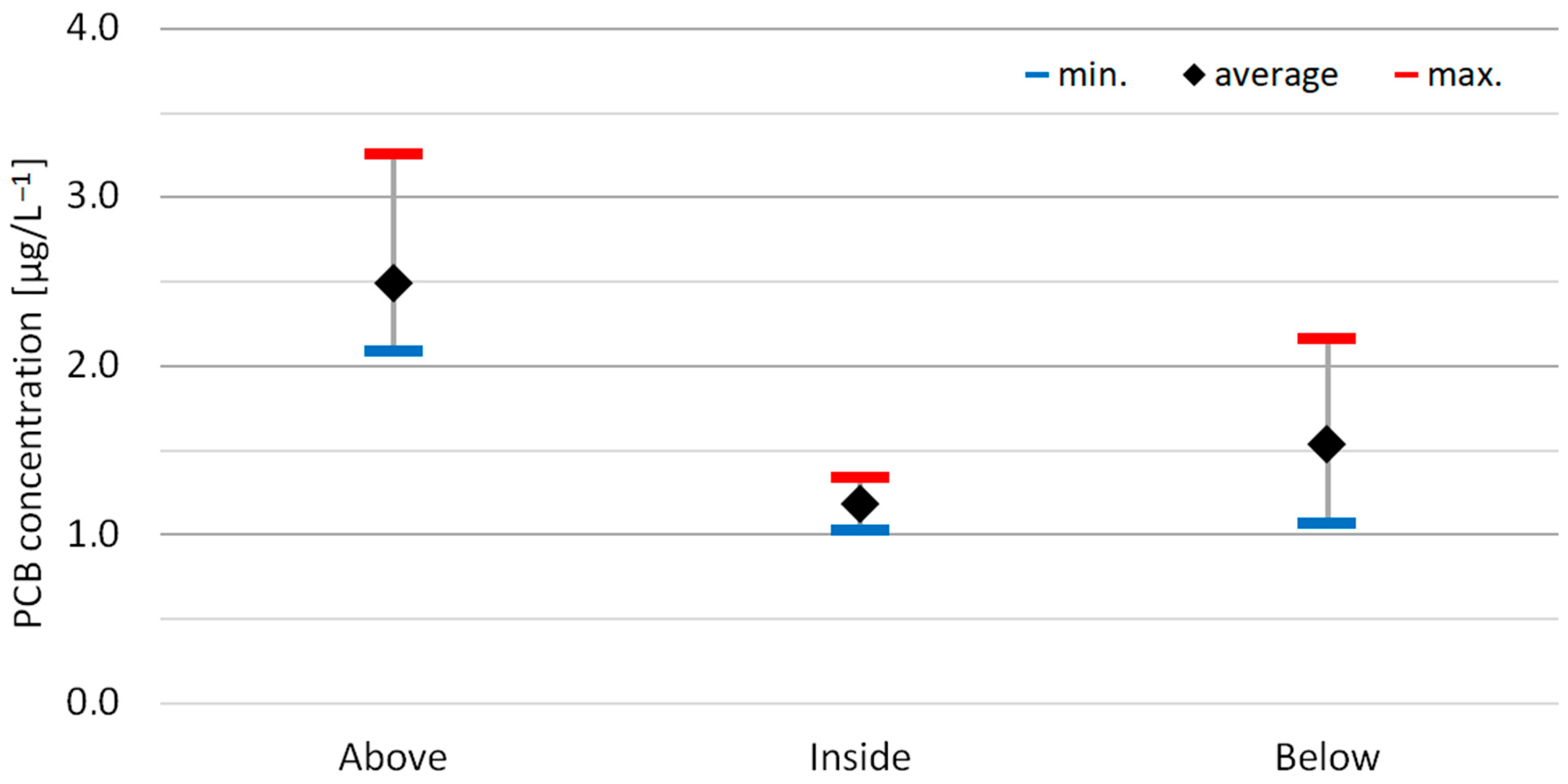

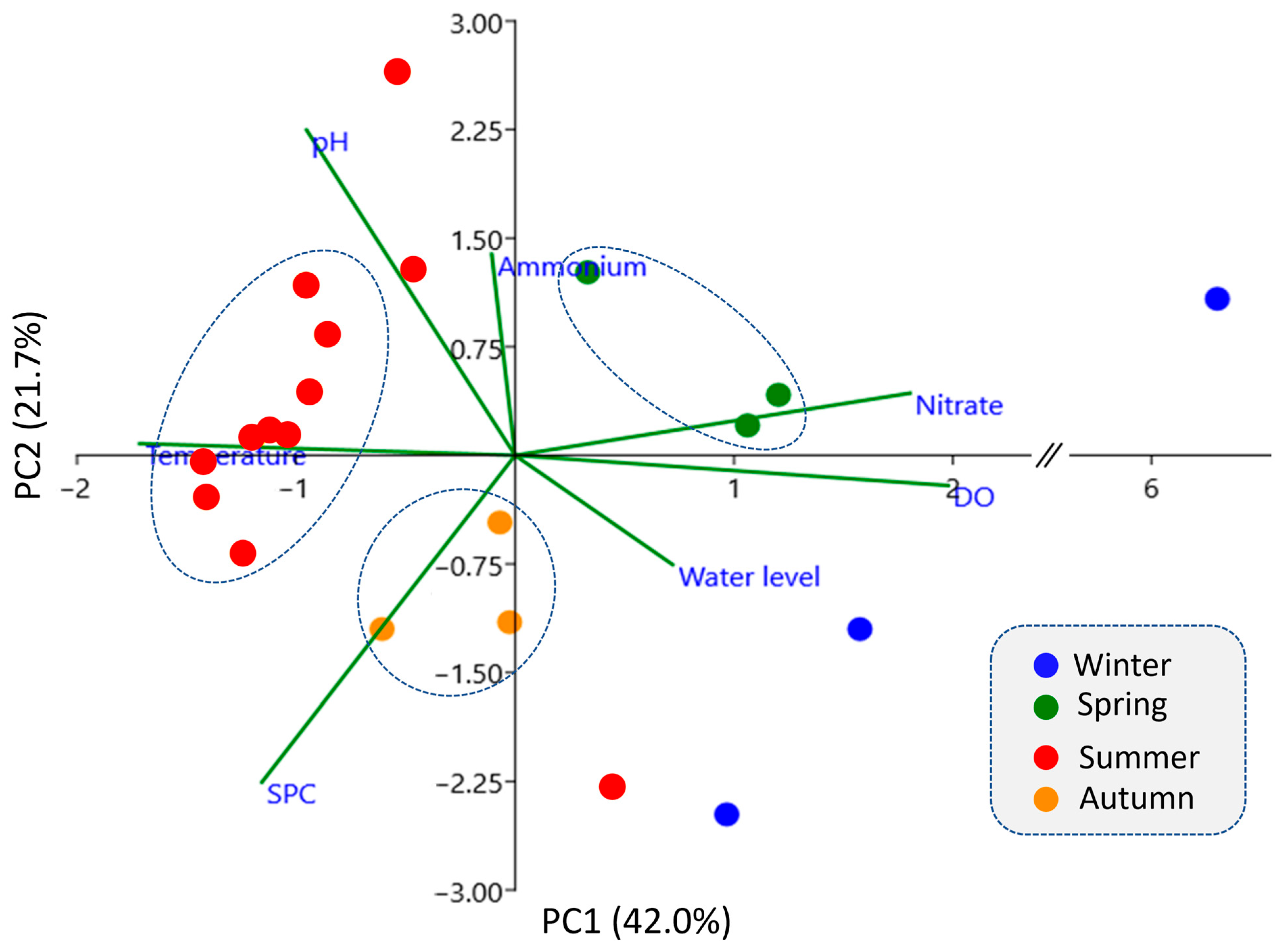
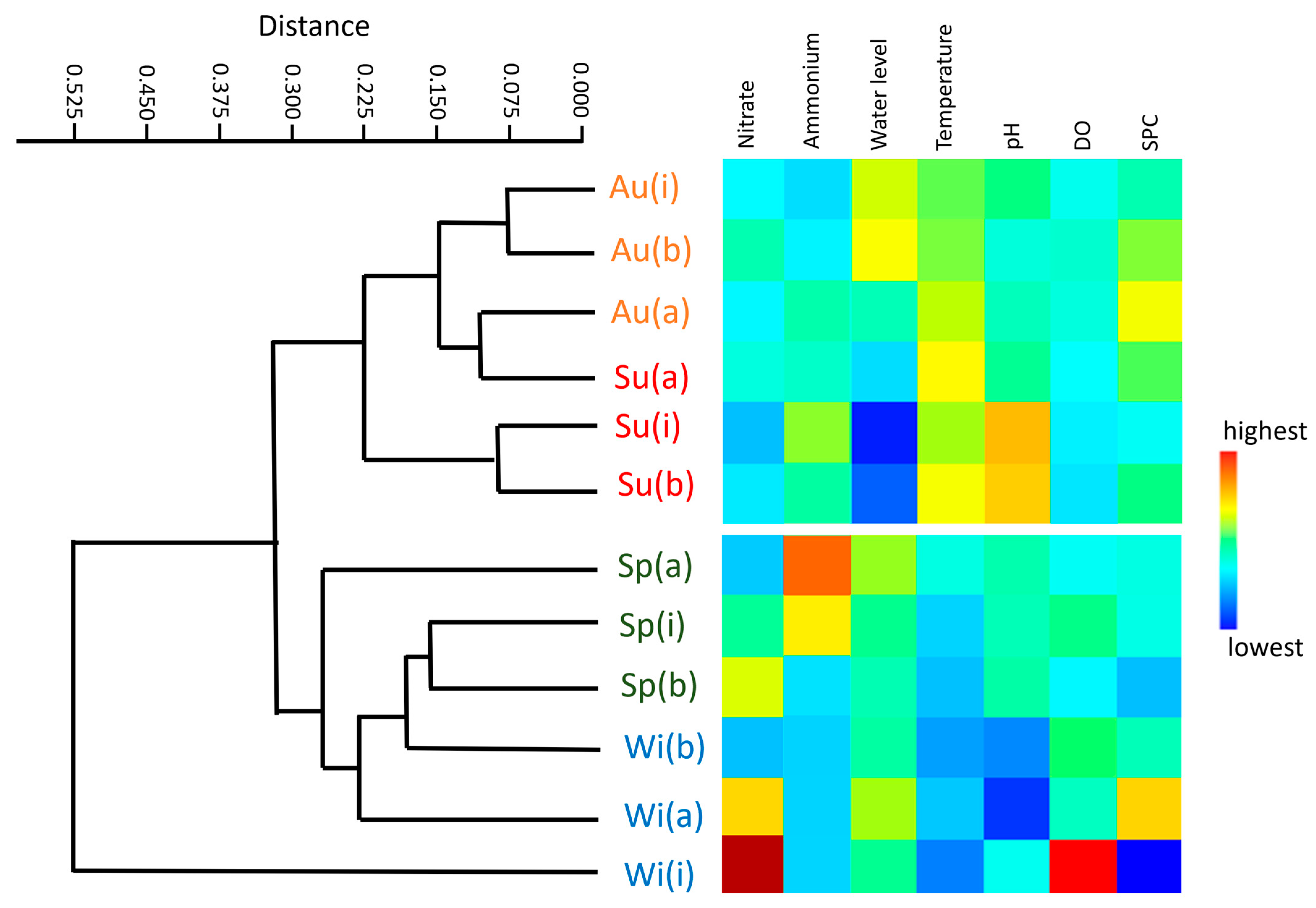
| Parameters | Above | Inside | Below |
|---|---|---|---|
| Water level [m]—Average ± SD (Minimum—Maximum) | 1.10 ± 0.27 (0.23–1.23) | 1.09 ± 0.24 (0.60–1.76) | 1.10 ± 0.20 (0.62–1.50) |
| Temperature [°C]—Average ± SD (Minimum—Maximum) | 15.16 ± 4.02 (6.00–20.80) | 14.08 ± 4.78 (1.90–20.60) | 12.73 ± 4.21 (4.30–18.70) |
| pH—Average ± SD (Minimum—Maximum) | 7.81 ± 0.63 (6.35–9.01) | 7.76 ± 0.80 (4.50–9.31) | 7.97 ± 0.64 (6.65–9.15) |
| Dissolved oxygen (DO) [mg·L−1]—Average ± SD (Minimum—Maximum) | 1.38 ± 1.12 (0.29–5.00) | 1.54 ± 1.23 (0.21–4.60) | 2.10 ± 2.10 (0.17–9.39) |
| Specific electrical conductivity (SEC) [μS cm−1]—Average ± SD (Minimum—Maximum) | 3925 ± 2586 (133–10,666) | 4365 ± 1804 (700–8408) | 2928 ± 1614 (680–7574) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupiec, J.M.; Szklarek, S.; Urbaniak, M.; Font-Nájera, A.; Mierzejewska-Sinner, E.; Bednarek, A.; Wójcik, J.; Mankiewicz-Boczek, J. Permeable Organic Barriers as Effective Tools for Reducing Emissions of Nitrogen Compounds and PCBs from Manure to Groundwater. Nitrogen 2025, 6, 105. https://doi.org/10.3390/nitrogen6040105
Kupiec JM, Szklarek S, Urbaniak M, Font-Nájera A, Mierzejewska-Sinner E, Bednarek A, Wójcik J, Mankiewicz-Boczek J. Permeable Organic Barriers as Effective Tools for Reducing Emissions of Nitrogen Compounds and PCBs from Manure to Groundwater. Nitrogen. 2025; 6(4):105. https://doi.org/10.3390/nitrogen6040105
Chicago/Turabian StyleKupiec, Jerzy Mirosław, Sebastian Szklarek, Magdalena Urbaniak, Arnoldo Font-Nájera, Elżbieta Mierzejewska-Sinner, Agnieszka Bednarek, Jakub Wójcik, and Joanna Mankiewicz-Boczek. 2025. "Permeable Organic Barriers as Effective Tools for Reducing Emissions of Nitrogen Compounds and PCBs from Manure to Groundwater" Nitrogen 6, no. 4: 105. https://doi.org/10.3390/nitrogen6040105
APA StyleKupiec, J. M., Szklarek, S., Urbaniak, M., Font-Nájera, A., Mierzejewska-Sinner, E., Bednarek, A., Wójcik, J., & Mankiewicz-Boczek, J. (2025). Permeable Organic Barriers as Effective Tools for Reducing Emissions of Nitrogen Compounds and PCBs from Manure to Groundwater. Nitrogen, 6(4), 105. https://doi.org/10.3390/nitrogen6040105








