1. Introduction
In a context of growing food demand and scarce natural resources, improving nitrogen use efficiency (NUE) is one of the main challenges for sustainable agriculture. Significant nitrogen losses through leaching, volatilization, and denitrification not only reduce fertilizer efficiency but also contribute to aquifer contamination, nitrogen oxide emissions, and soil quality deterioration [
1]. Despite advances in agricultural intensification, nitrogen use efficiency (NUE) remains low in many cropping systems worldwide, reflecting inefficient uptake and utilization of applied nitrogen and resulting in significant environmental losses, such as nitrate leaching and greenhouse gas emissions [
2]. Nitrogen is an essential macronutrient involved in fundamental processes such as the biosynthesis of amino acids, proteins, nucleic acids, and chlorophyll, and directly affects photosynthesis, growth, reproduction, and crop yield [
3,
4]. Both its deficiency and excess negatively affect agronomic performance and ecological balance.
In recent years, some micronutrients have attracted interest for their potential to modulate nitrogen-related metabolic pathways, notably iodine, an element that has historically been underestimated in plant physiology. Previous studies have documented the biostimulant and antioxidant effects of iodine in different species [
5,
6]. In particular, foliar application of potassium iodide (KI) has been shown to increase chlorophyll and carotenoid content, improve antioxidant capacity by increasing ascorbic acid and phenolic compounds, and promote plant growth and fresh biomass accumulation in crops such as lettuce and strawberry under hydroponic conditions [
7,
8]. However, the effectiveness of iodine may be limited by its high mobility in the soil and its volatilization in the form of organic compounds, which reduces its availability to plants [
9].
Given the limitations of conventional iodine fertilization—particularly its high mobility in soil and susceptibility to volatilization—iodine nanoparticles (INPs) emerge as a promising strategy to enhance the stability, bioavailability, and physiological performance of iodine in plants. Nanotechnology enables controlled release, minimizes nutrient losses, and increases absorption efficiency through greater cellular interaction [
10,
11]. While INPs have not yet been directly tested in agricultural systems, studies using conventional iodine forms (iodide or iodate) have reported improvements in plant growth, antioxidant capacity, and nitrogen-related metabolism [
12,
13,
14]. These findings provide a strong rationale for developing iodine-based nanofertilizers. Based on this premise, the present study evaluates INPs as an innovative approach to enhance iodine efficiency and physiological responses in plants, under the hypothesis that nanoformulation can overcome key agronomic limitations of traditional iodine sources.
However, there is still little comparative evidence on the physiological efficacy of iodine nanoparticles (INP) versus traditional iodine sources, such as KI, in horticultural crops, especially with regard to nitrogen metabolism indicators, such as nitrate reductase (NR) activity, amino acid accumulation, protein content, chlorophyll concentration, SPAD index, biomass, and yield. Although INPs have not yet been tested directly in cropping systems, several studies with iodine-containing nanoformulations or foliar applications of iodine salts suggest a possible relationship between iodine treatments and improved metabolic responses. For example, the combined application of iodide and selenate improved biofortification and nitrogen-related metabolism in chervil, even without the use of nanoparticles [
15]. In tomatoes, chitosan and iodine complexes applied foliar improved antioxidant metabolism, which may indirectly favor nitrogen use efficiency under stress conditions [
16]. Similarly, foliar application of iodate salts to spinach and lettuce under salt stress influenced mineral uptake, reduced nitrate accumulation, and improved antioxidant status, effects that may be associated with improved nitrogen metabolism [
17,
18]. Although these studies do not include iodine nanoparticles per se, they provide indirect evidence supporting the rationale for evaluating INPs as a next-generation iodine delivery system in agriculture. Given the absence of previous experimental data on INPs in agriculture, this study provides the first comparative evaluation of their performance versus conventional iodine sources in a horticultural crop.
Therefore, the objective of this study was to compare the efficacy of iodine nanoparticles (INPs) versus potassium iodide (KI), both applied via foliar application, on nitrogen assimilation, biomass, and yield in Lactuca sativa. The hypothesis was that INPs induce greater improvements in nitrogen-related metabolic processes and agronomic performance due to their greater stability and physiological efficiency.
2. Materials and Methods
2.1. Experimental Sites and Crop Management
The experiment was conducted between March and May 2024 at the Center for Food and Development Research (CIAD), Delicias Unit, Chihuahua, Mexico, under controlled conditions in a greenhouse with shade netting. Seeds of Lactuca sativa L. cv. Butterhead, purchased from Islas’s Garden Seed Company® (Eagle Rd, ID, USA), were used. Seeds were sown in polystyrene trays using vermiculite as substrate, and during the first 30 days, 250 mL of a modified Hoagland nutrient solution was applied every three days.
The solution formulation was based on that proposed by Sánchez et al. [
19], consisting of: 6 mM NH
4NO
3, 1.6 mM K
2HPO
4, 2.4 mM K
2SO
4, 4 mM CaCl
2·2H
2O, 1.4 mM MgSO
4·H
2O, 5 µM Fe-EDDHA, 2 µM MnSO
4·H
2O, 1 µM ZnSO
4·7H
2O, 0.25 µM CuSO
4·5H
2O, 0.3 µM Na
2MoO
4·2H
2O, and 25 µM H
3BO
3; with pH adjusted to 6.0 ± 0.1 and renewed every 72 h. The solution used provided potassium through K
2SO
4 and K
2HPO
4, ensuring a sufficient concentration of K
+ to meet the nutritional demands of the plant in all treatments.
Thirty days after sowing (DAS), the seedlings were transplanted into 5 L plastic pots, using a mixture of vermiculite and perlite in a 2:1 (
v/
v) ratio as substrate. The irrigation system used was passive hydroponics by sub-irrigation, in which the pots were placed on plastic trays (33 cm × 55 cm × 4 cm) with a constant layer of nutrient solution. This method favors the rise in water and nutrients by capillary action from the base of each pot, ensuring a uniform and efficient supply without wetting the foliage [
20].
From transplanting until physiological maturity (41 DAS), and subsequently until harvest (71 DAS), 1 L of nutrient solution was supplied daily per pot, maintaining the pH at 6.0 ± 0.1.
2.2. Characterization of Iodine Nanopartices (INPs)
The iodine nanoparticles (INPs) used in this study were synthesized and supplied by Investigación y Desarrollo de Nanomateriales S.A. de C.V. (San Luis Potosí, Mexico). According to the technical data sheet, INPs were obtained through a wet chemical synthesis process involving reduction reactions from potassium iodate precursors under controlled temperature and agitation, resulting in a fine dark brown powder.
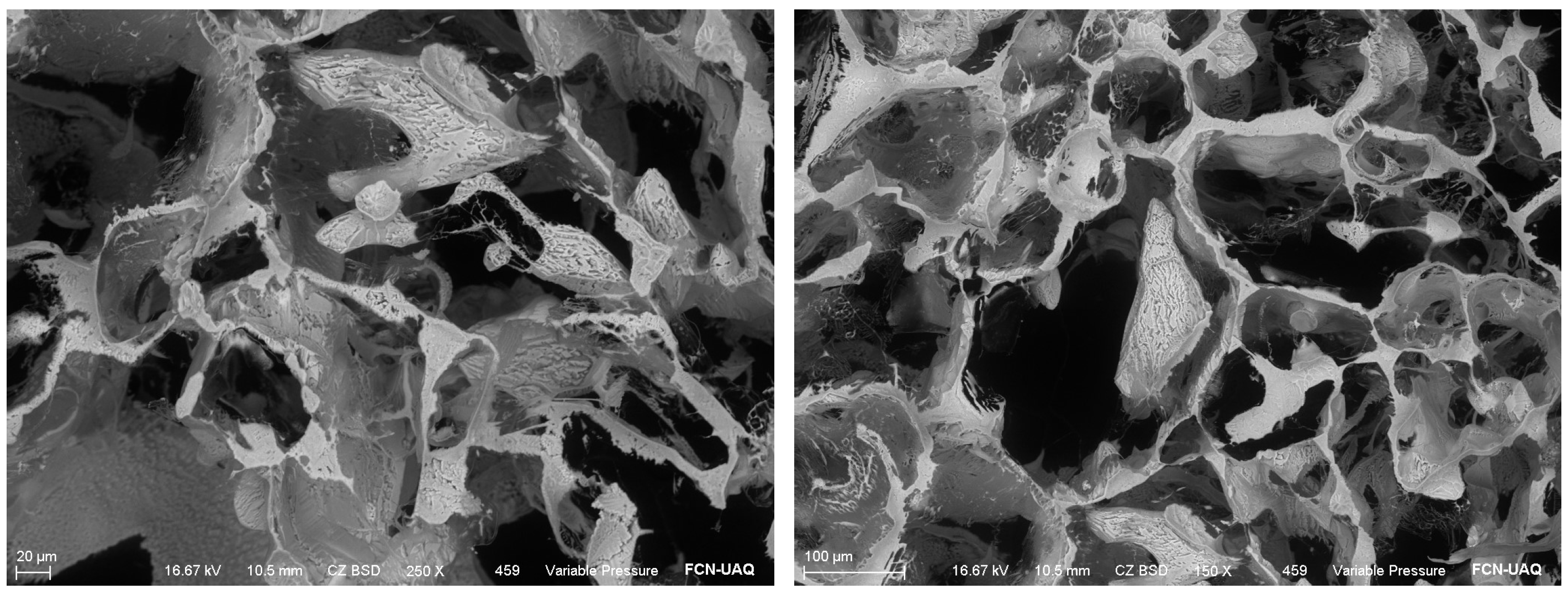
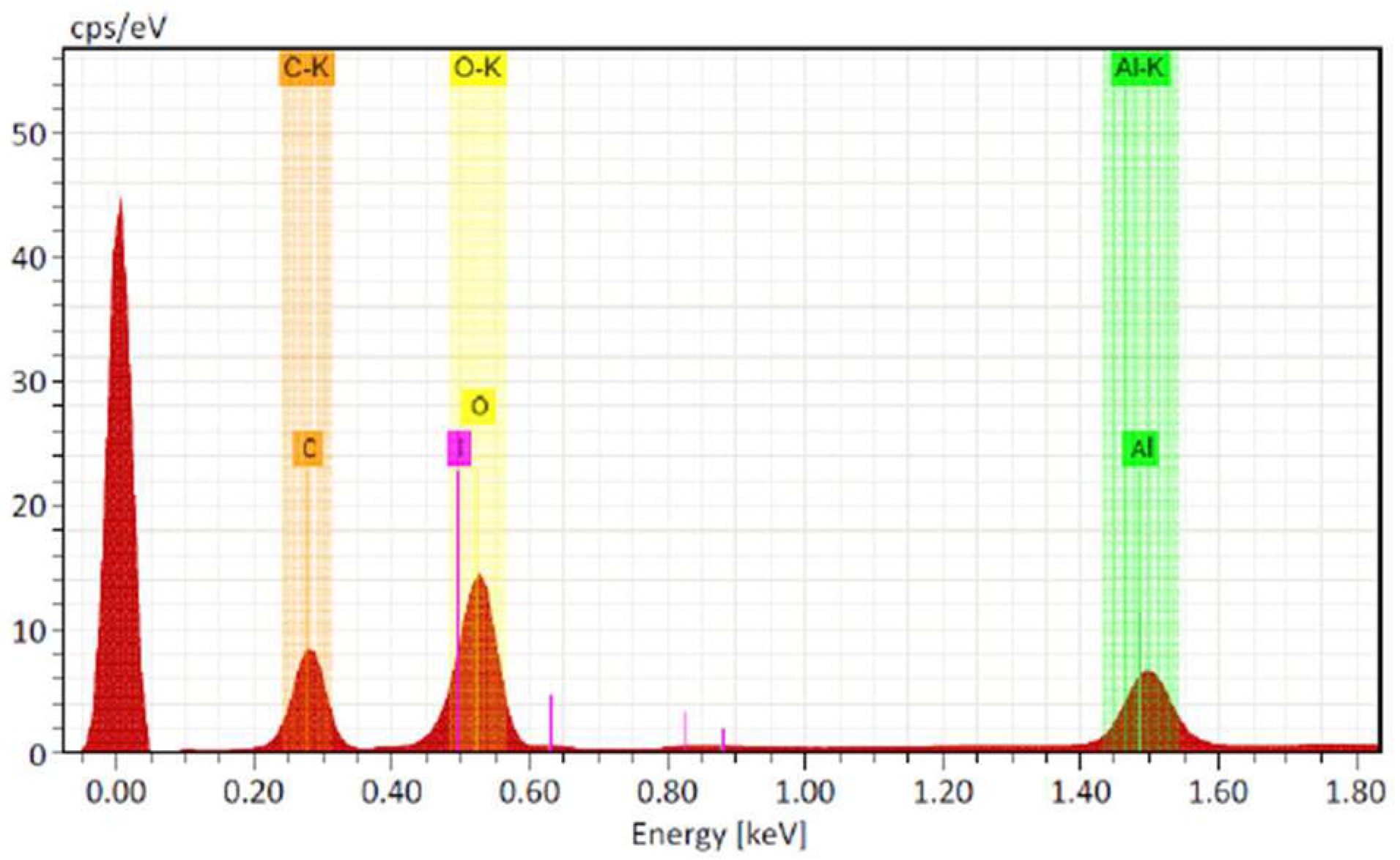
Structural and morphological characterization was performed by the manufacturer using scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS), which confirmed the presence of elemental iodine and showed spherical and irregular particles partially agglomerated with sizes ranging from 80 to 150 nm (
Figure 1 and
Figure 2). The product had an iodine purity of 97.3%, a pH of 7.1 (1% solution), and an apparent density of 0.75 g cm
−3.
Due to the limited literature on the physiological role of iodine nanoparticles in plants, the concentrations applied in this study were determined based on equivalence with potassium iodate used in agricultural research [
21].
2.3. Preparation of Iodine Nanoparticles Suspensions
A stock suspension of iodine nanoparticles (INPs) was prepared at a concentration of 320 µM by dispersing the nanoparticle powder (supplied by Investigación y Desarrollo de Nanomateriales S.A. de C.V., San Luis Potosí, Mexico) in distilled water three times. The suspension was homogenized by mechanical stirring on a magnetic plate (VWR
®, Radnor, PA, USA) at 700 rpm for 20 min, followed by sonication in an ultrasonic bath (Vevor Ultrasonic Cleaner, Cleveland, OH, USA) at 40 kHz for 15 min, at a controlled temperature of 25–30 °C. This procedure was adapted from the method described by Mazhar et al. [
22]. From this stock suspension, serial dilutions were prepared to obtain the final concentrations used in the experimental treatments.
2.4. Experimental Design and Treatments
A completely randomized design with a single-factor layout consisting of six treatments was used, resulting from the combination of two iodine sources (potassium iodide KI and iodine nanoparticles INPs) applied in three concentrations: 40, 80, and 160 μM. The treatments established were Control (0 μM), KI 40 μM, KI 80 μM, KI 160 μM, INP 40 μM, INP 80 μM, and INP 160 μM. Each treatment included four replicates, and the experimental unit consisted of one plant per pot. Four replicates per treatment were used due to space constraints within the controlled environment facility, which limited the number of experimental units that could be maintained under homogeneous environmental conditions. However, each plant was grown individually in separate pots and received standardized and strictly monitored inputs, ensuring rigorous experimental control.
Foliar applications were carried out using a pressurized manual sprayer until runoff, with a volume of approximately 50 mL per plant. The treatments were applied on days 45, 52, 59, and 66 DAS under controlled environmental conditions.
2.5. Plant Sampling
Once the plants reached physiological maturity at 71 DAS, the plant material was harvested. The collected material was washed with distilled water to remove residues and finally separated into organs (root, leaves). The samples were divided into fresh and dry material. The fresh material was used to determine yield, nitrate reductase enzyme activity, and the concentration of photosynthetic pigments, amino acids, and soluble proteins. The dry material was used to quantify biomass.
2.6. Plant Analysis
2.6.1. Total Biomass and Yield
To quantify total biomass and yield, one plant was randomly selected from each pot and weighed fresh using a compact balance (A&D Co., Ltd., EK-120, Tokyo, Japan). The plant was then dissected into leaves and roots, and each organ was weighed fresh. Yield was expressed as the fresh weight of leaves per plant (g plant−1 FW).
The organs obtained were rinsed three times in distilled water and dried on filter paper at room temperature for 24 h. After this period, 50% of the plant material was dried in a 13.9 cubic foot forced-air laboratory oven (Shel-Lab 1380FX, Cornelius, OR, USA) at 45 °C for 72 h. Once the samples had lost moisture, they were weighed using an electronic analytical balance (A&D Co., Ltd., HR-120, Tokyo, Japan). Total biomass was expressed as the sum of the dry weight of the two plant organs (g plant−1 DW).
2.6.2. Extraction and Assay of NR (E.C. 1.7.1.1)
For each sampling, 100 mg of fresh root or leaf tissue were homogenized in a chilled mortar at 0 °C in a 1:5 (w/v) ratio using 50 mM potassium phosphate buffer (KH2PO4, pH 7.5, Sigma-Aldrich, ≥99%, Toluca de Lerdo, State of Mexico, Mexico). The extraction buffer included 2 mM ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich, ≥99.4%), 1.5% (w/v) soluble casein (Merck, ≥90%, Naucalpan, State of Mexico, Mexico), 2 mM dithiothreitol (DTT, Sigma-Aldrich, ≥99%), and 1% (w/v) polyvinylpolypyrrolidone (PVPP, Sigma-Aldrich, analytical grade). The homogenate was filtered through two layers of Miracloth (Calbiochem, Toluca de Lerdo, State of Mexico, Mexico) and centrifuged at 3000× g for 5 min at 4 °C using a refrigerated centrifuge (Allegra™, 64R Centrifuge, Beckman Coulter, Brea, CA, USA). The resulting supernatant was then centrifuged again at 30,000× g for 20 min under the same conditions. The final supernatant was used to determine enzymatic activities and soluble protein content.
Nitrate reductase (NR) activity was assayed using the method of Kaiser and Lewis [
23], in which nitrate is reduced in the presence of NADH and the nitrite formed is quantified. The nitrite was colorimetrically determined at 540 nm after diazotization with sulphanilamide (Sigma-Aldrich, ≥98%) and
N-(1-naphthyl)ethylenediamine dihydrochloride (Merck, ≥98%), according to the method of Hageman and Hucklesby [
24]. Enzyme activity was expressed as μmol NO
2− formed per milligram of protein per minute (μmol NO
2− mg
−1 protein min
−1).
2.6.3. Photosynthetic Pigments
The concentrations of chlorophyll a, b and total were determined using the method described by Wellburn [
25]. Ten leaf discs with a diameter of 7 mm were weighed and infiltrated in 10 mL of methanol (CH
3OH). The samples were sealed and left to stand in the dark for 24 h. After this time, the absorbance of the samples was measured at wavelengths of 653 and 666 nm for chlorophyll b and chlorophyll a, respectively, using a UV-visible spectrophotometer (Thermo Fisher Scientific, GENESYS™ 10S, Madison, WI, USA). Pigment concentrations were expressed in μg cm
2 FW and calculated using the following formulas:
where Chl a* and Chl b*: are intermediate values from absorbance; Chl a and Chl b: are final pigment concentrations in μg cm
−2 FW; A: absorbance; Vf: final volume; W1: weight per leaf disc; W2: total weight of leaf discs; r: radius of leaf discs; n: number of leaf discs.
2.6.4. Concentrations of Soluble Amino Acids and Soluble Proteins
An amount of 0.5 g of leaf blade plant sample was homogenized with 5 mL of 50 mM phosphate buffer, pH = 7 at 4 °C (solution of 6.8 g of K
2HPO
4 dissolved in 1 L of distilled water, adjusted to pH = 7 with a solution of 8.81 g KH
2PO
4 dissolved in 1 L of distilled water). The sample was filtered through 4 layers of gauze and centrifuged at 10,000 rpm for 15 min in a centrifuge cooled to 4 °C (Allegra™, 64R Centrifuge, Beckman Coulter, Brea, CA, USA). The supernatant was used to determine the concentrations of amino acids and soluble proteins using the methods described by Yemm et al. [
26] and Bradford [
27], respectively.
To quantify soluble amino acids, an aliquot of 100 μL of supernatant was mixed with 1.5 mL of ninhydrin reagent (2 g of ninhydrin dissolved in 50 mL of ethylene glycol (CH2OHCH2OH), mixed with 80 mg of stannous chloride (SnCl2·2H2O), dissolved in 50 mL of 200 mM citrate buffer at pH = 5 (solution of 59.41 g of tribasic sodium citrate dihydrate (C6H5Na3O72H2O), dissolved in 1 L of distilled water, and buffered to pH 5 with a solution of 38.81 g of anhydrous citric acid (C6H8O7). The sample was stirred and placed in a water bath at 100 °C for 20 min. The samples were then placed in a water bath at 4 °C for 30 min and reacted with 8 mL of 50% (v/v) 1-propanol (C3H8O). Finally, the samples were measured at 570 nm using a UV-visible spectrophotometer (Thermo Fisher Scientific, GENESYS™ 10S, Madison, WI, USA) against a glycine standard curve. The results were expressed as (mg g−1 FW).
For the quantification of soluble proteins, a 20 μL aliquot of the centrifuged supernatant was taken and mixed with 1 mL of the Bradford Quick Start™ Protein Assay Kit (Bio-rad, Hercules, CA, USA) coloring reagent. The samples were shaken and allowed to stand for 15 min. Finally, they were measured at an absorbance of 595 nm using a UV-visible spectrophotometer (Thermo Fisher Scientific, GENESYS™ 10S, Madison, WI, USA) and compared to a standard curve of bovine serum albumin (BSA).
The curve was prepared by taking 20 μL of each of the standards from the Bradford Quick Start™ protein assay kit at concentrations of 0.125, 0.25, 0.5, 0.75, 1, 1.5, and 2 mg mL−1 of BSA and distilled water for the blank. The results were expressed as (mg g−1 FW).
2.6.5. Pearson Correlation Heatmap
To evaluate the relationships between physiological and biochemical variables, a Pearson correlation matrix was developed using the averages per treatment. The results were represented graphically using a heatmap generated in Python (v 3.11), where the intensity and direction of the correlations were expressed using a color scale. Correlation values close to +1 indicate a strong positive association, while values close to −1 reflect a strong negative correlation. Correlations close to 0 are interpreted as weak or non-existent, allowing functional or independent relationships between the evaluated variables to be identified [
28].
2.7. Statistical Analysis
Statistical analysis was based on a linear fixed-effects model corresponding to a completely randomized design with a single factor, expressed by Equation (4):
where Yij was the response variable, under the effect of the i
th treatment and the j
th repetition; μ was the overall mean; τi was the effect of the i
th treatment; and εij was the experimental error.
Prior to analysis of variance (ANOVA), the data were evaluated to verify compliance with the assumptions of normality and homogeneity of variances. Normality was tested using the Shapiro–Wilk test, while homogeneity of variances was evaluated using Bartlett’s test. Once compliance with these assumptions was confirmed, a one-way ANOVA was performed.
When statistically significant differences were detected between treatments (
p ≤ 0.05), a mean comparison test was applied using Fisher’s Least Significant Difference (LSD) method, with a significance level of
p ≤ 0.05. The analyses were performed using SAS
® statistical software version 9.0 (SAS Institute Inc., Cary, NC, USA) [
29]. Treatments with different letters indicated statistically significant differences according to the LSD test.
3. Results and Discussion
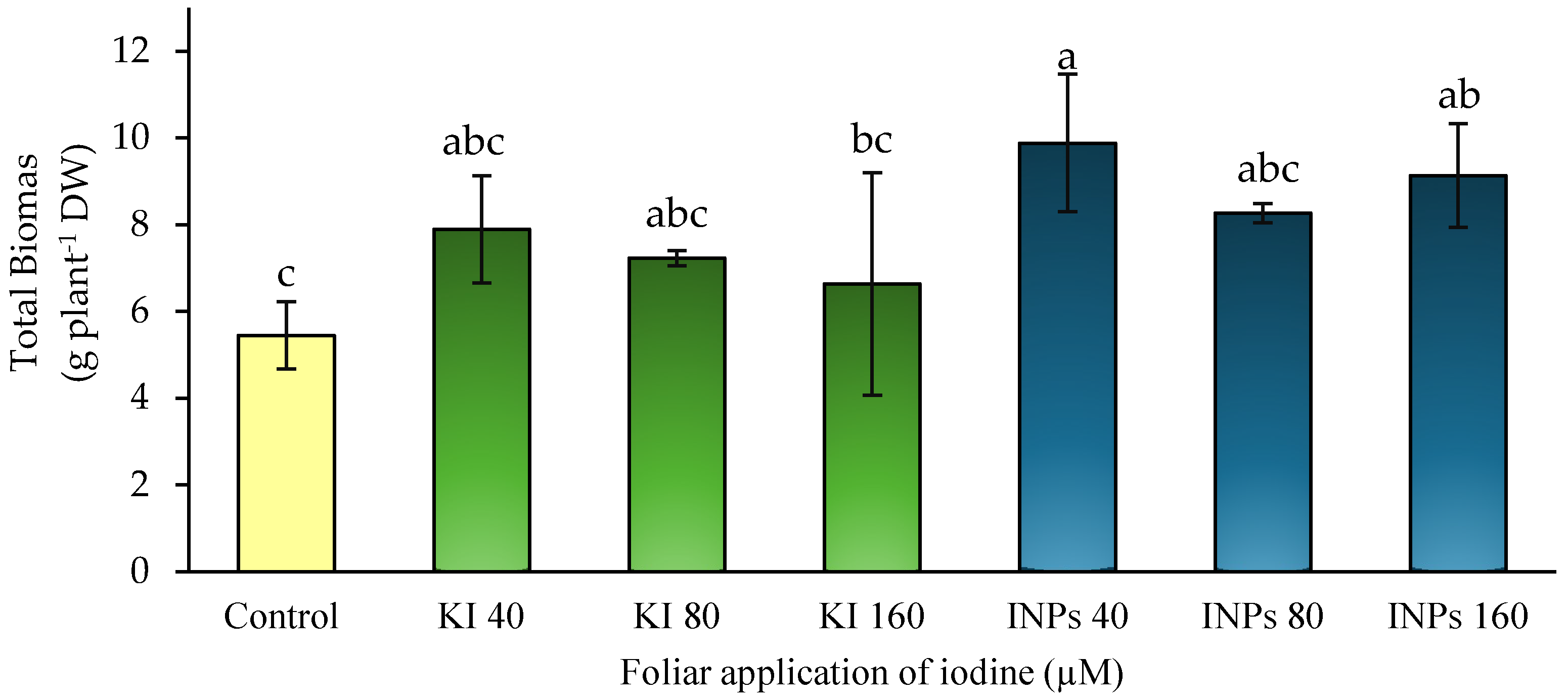
3.1. Total Biomass
Biomass accumulation is a comprehensive indicator of plant growth and reflects the functional efficiency of nitrogen metabolism, as nitrogen is actively involved in the synthesis of nucleic acids, proteins, and chlorophyll, processes that are essential for plant development and yield formation [
3]. In the present study, significant differences (
p ≤ 0.05) in total dry biomass were observed between treatments (
Figure 3).
It is noteworthy that foliar application of iodine nanoparticles (INP) at 40 μM caused a 68.75% increase in biomass compared to the untreated control. In contrast, treatments with potassium iodide (KI) at the same dose did not result in statistically significant increases. These findings suggest a distinctive physiological effect that could be attributed to the nanostructured nature of iodine, although the precise mechanisms remain to be clarified.
The literature indicates that the effect of iodine on plant biomass is influenced by multiple factors, such as concentration, chemical form, application method, and cultivar sensitivity [
30,
31]. For example, Medrano-Macías et al. [
6] summarized several studies and noted that potassium iodate, especially when applied in combination with nitrogen, increased lettuce biomass under greenhouse conditions. Similarly, Riyazuddin et al. [
32] documented that iodine fertilization could act as a growth promoter in horticultural crops, especially under stress conditions, by influencing redox homeostasis and nutrient assimilation pathways.
Although none of these studies refer to iodine nanoparticles, several reports on artificial nanomaterials have shown that nanostructures can improve nutrient absorption, cellular uptake, and intracellular mobility of active compounds [
33,
34]. These effects are often attributed to the small size, large surface-to-volume ratio, and controlled release behavior of nanoparticles [
35,
36]. Although there is currently no direct evidence that INPs modulate nitrogen-related enzymes, the general role of nanoparticles in facilitating nutrient metabolism, including nitrogen assimilation, has been described for other nanomaterials [
11].
It is also important to note that excessive concentrations of iodine, especially in sensitive genotypes, can cause phytotoxicity, which manifests as leaf chlorosis, necrosis, or growth inhibition. These effects are associated with the overproduction of reactive oxygen species (ROS) and redox imbalance in plant tissues [
5,
37]. In this study, none of these toxicity symptoms were observed at the concentrations tested, indicating that 40 μM INP is within a physiologically safe range for
Lactuca sativa. 3.2. Yield
Agricultural yield is an integrative indicator of plant physiological functioning, as it reflects the efficiency with which plants convert resources, especially assimilable nitrogen, into reproductive biomass. Since nitrogen is essential for the synthesis of amino acids, structural proteins, and enzymes associated with floral development and harvest organ filling, yield allows us to indirectly infer the functional state of nitrogen metabolism [
3].
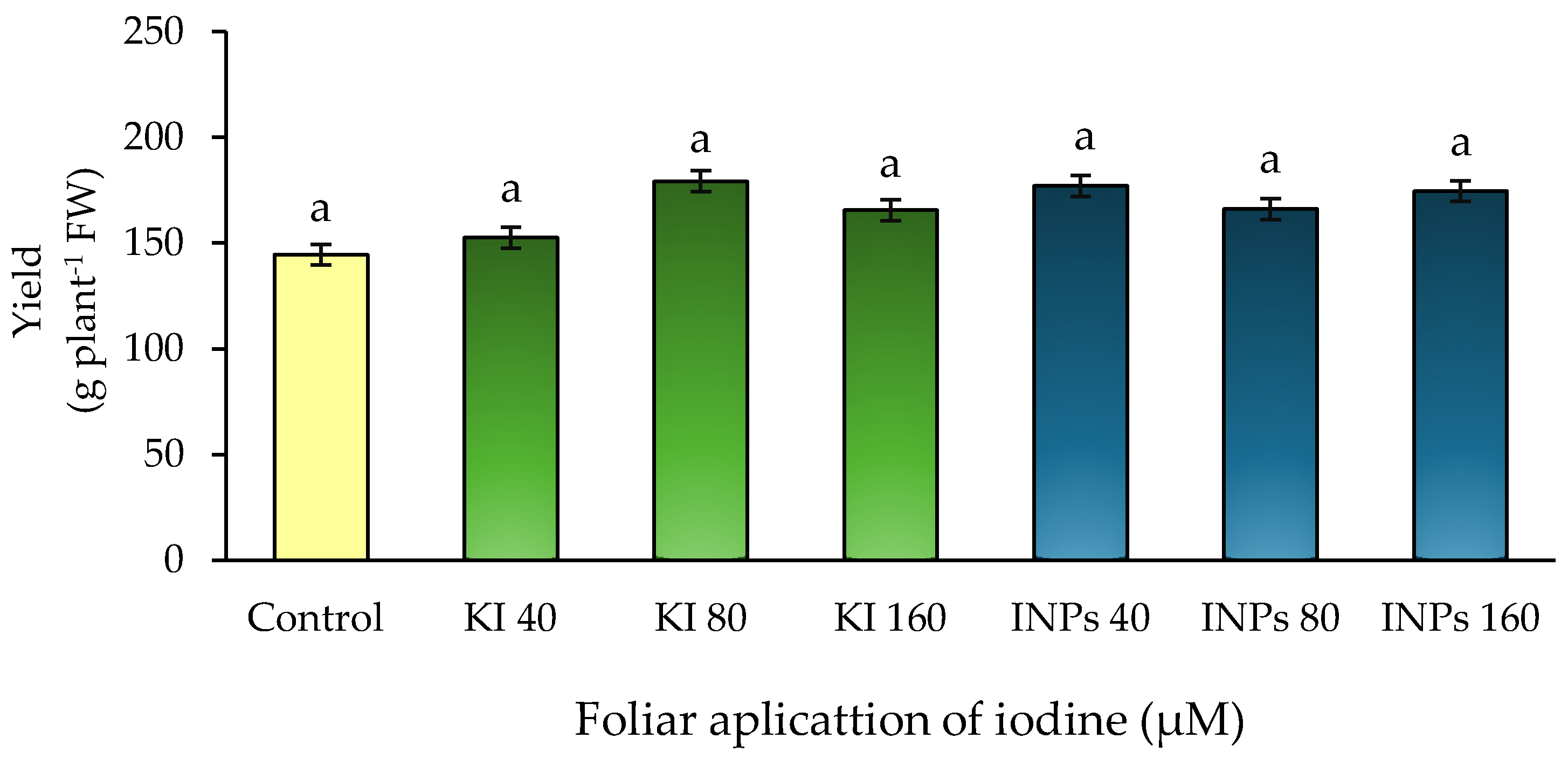
In the present study, no statistically significant differences (
p > 0.05) in fresh yield were observed between treatments with potassium iodide (KI) or iodine nanoparticles (INP), regardless of the concentration applied (
Figure 4). Although slight numerical variations were recorded, these were not dose-dependent and did not follow a consistent physiological trend, preventing firm conclusions about yield increase.
These findings are particularly noteworthy when compared to the significant increase in total biomass observed in the INP-40 μM treatment (
Section 3.1), suggesting a disconnection between vegetative biomass production and commercial yield accumulation. This divergence may reflect a differential allocation of assimilates between leaf expansion and tissue density or water content, especially in the short growth cycles typical of
Lactuca sativa.
Previous studies investigating the effects of iodine fertilization on lettuce have predominantly used conventional forms of iodine, such as potassium iodate (KIO
3) or iodide salts (KI), applied foliar or to the soil. For example, Lawson et al. [
7] evaluated the application of iodine to both soil and leaves and observed no significant changes in yield, although iodine content in edible tissues increased considerably. It should be noted that their study did not include nanoparticulate formulations, and physiological responses were dose-dependent and cultivar-specific. Similarly, Duborská et al. [
38] reviewed multiple biofortification strategies with iodine and selenium in horticultural crops and did not observe consistent improvements in yield, emphasizing that the main effect was nutritional rather than agronomic.
In contrast, the present study is, to our knowledge, the first to explore the foliar application of iodine nanoparticles (INP) in lettuce. The absence of yield increase despite the increase in vegetative biomass underscores the need for further investigation of the physiological distribution of resources and the mode of action of nanostructured iodine, which may influence metabolic pathways involved in cell expansion or stress preparedness, rather than directly stimulating reproductive production or biomass compactness.
Therefore, although INPs demonstrated a biostimulant activity on vegetative growth without inducing any phytotoxic effects, this did not translate into higher fresh yield. These results confirm the agronomic safety of INPs and KI at the tested doses.
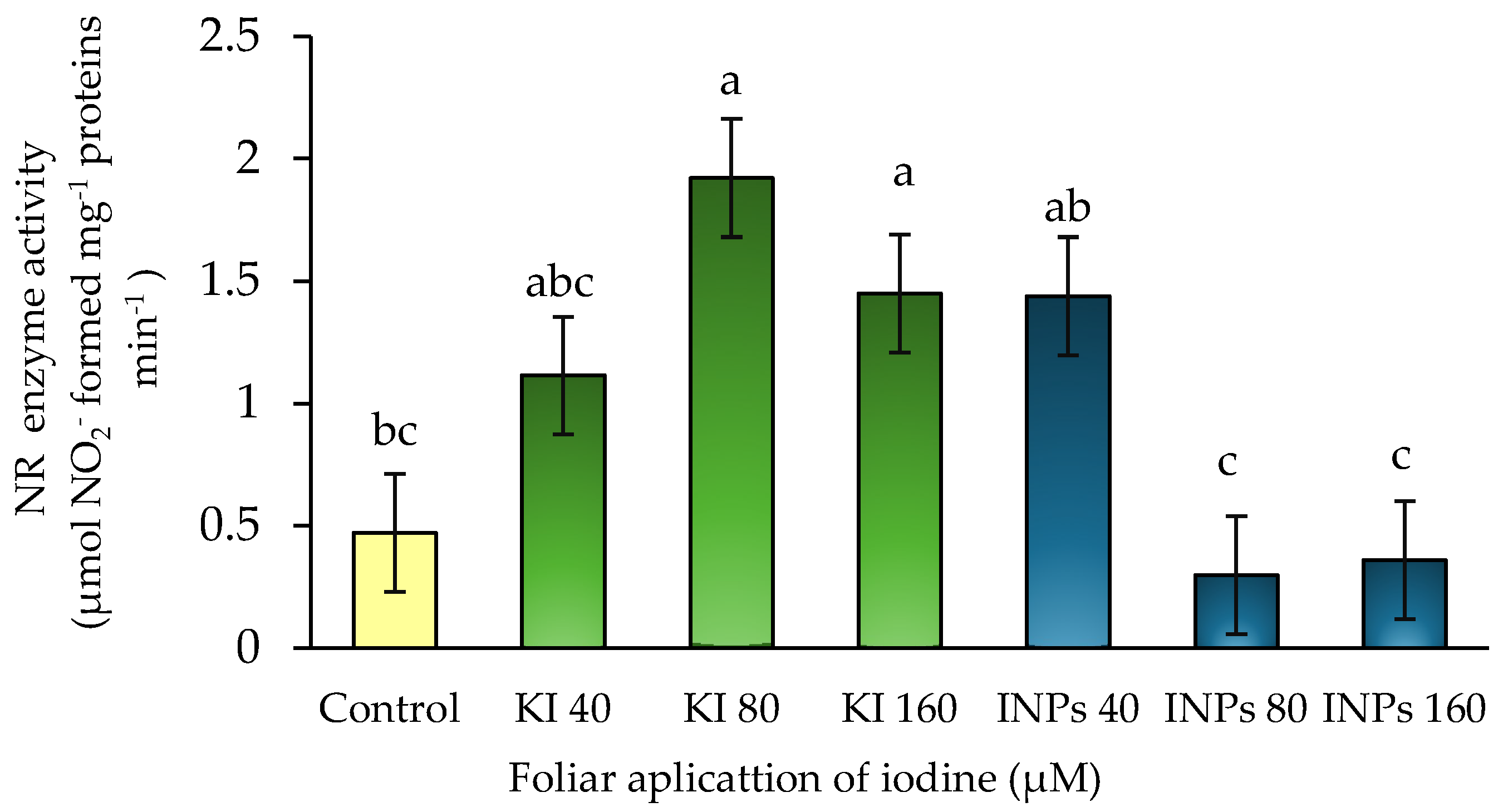
3.3. In Vivo Nitrate Reductase Enzyme Activity (NR) (E.C. 1.7.1.1)
Nitrate reductase (NR) catalyzes the first and rate-limiting step in nitrogen assimilation, converting nitrate (NO
3−) into nitrite (NO
2−), a process highly dependent on substrate availability and cellular redox conditions. Its activity is widely recognized as a functional indicator of nitrogen use efficiency in higher plants [
23].
In this study, statistically significant differences in NR activity (
p ≤ 0.05) were observed among treatments (
Figure 5). Foliar application of potassium iodide (KI) at 80 µM increased NR activity by 307% compared to the control, whereas treatments with iodine nanoparticles (INPs) at 80 and 160 µM led to reductions of 25% and 38%, respectively. This form-dependent effect of iodine aligns with previous findings. For instance, Silva-Marrufo et al. [
8] reported increased nitrogen content in hydroponic strawberry after KI foliar application, while Shalaby et al. [
18] observed reduced nitrate levels and elevated amino acid accumulation in lettuce, suggesting enhanced nitrogen assimilation. Smoleń and Sady [
30] also demonstrated that foliar-applied iodide improved nitrogen metabolism in spinach.
Our findings reinforce this evidence by showing that moderate foliar doses of KI effectively stimulate NR activity in lettuce. In contrast, INPs—despite being iodine carriers—exerted a detrimental effect. Their particulate nature may promote oxidative stress via reactive oxygen species (ROS) generation, disrupting intracellular redox homeostasis and impairing redox-sensitive enzymes like NR [
39]. Furthermore, their colloidal form might restrict iodine bioavailability in the cytosol, potentially due to sequestration in the apoplast or vacuoles.
This differential response highlights that the physiological effectiveness of iodine is governed not merely by concentration, but by its chemical form and its interaction with cellular redox dynamics, directly influencing nitrogen metabolism through enzymes such as NR.
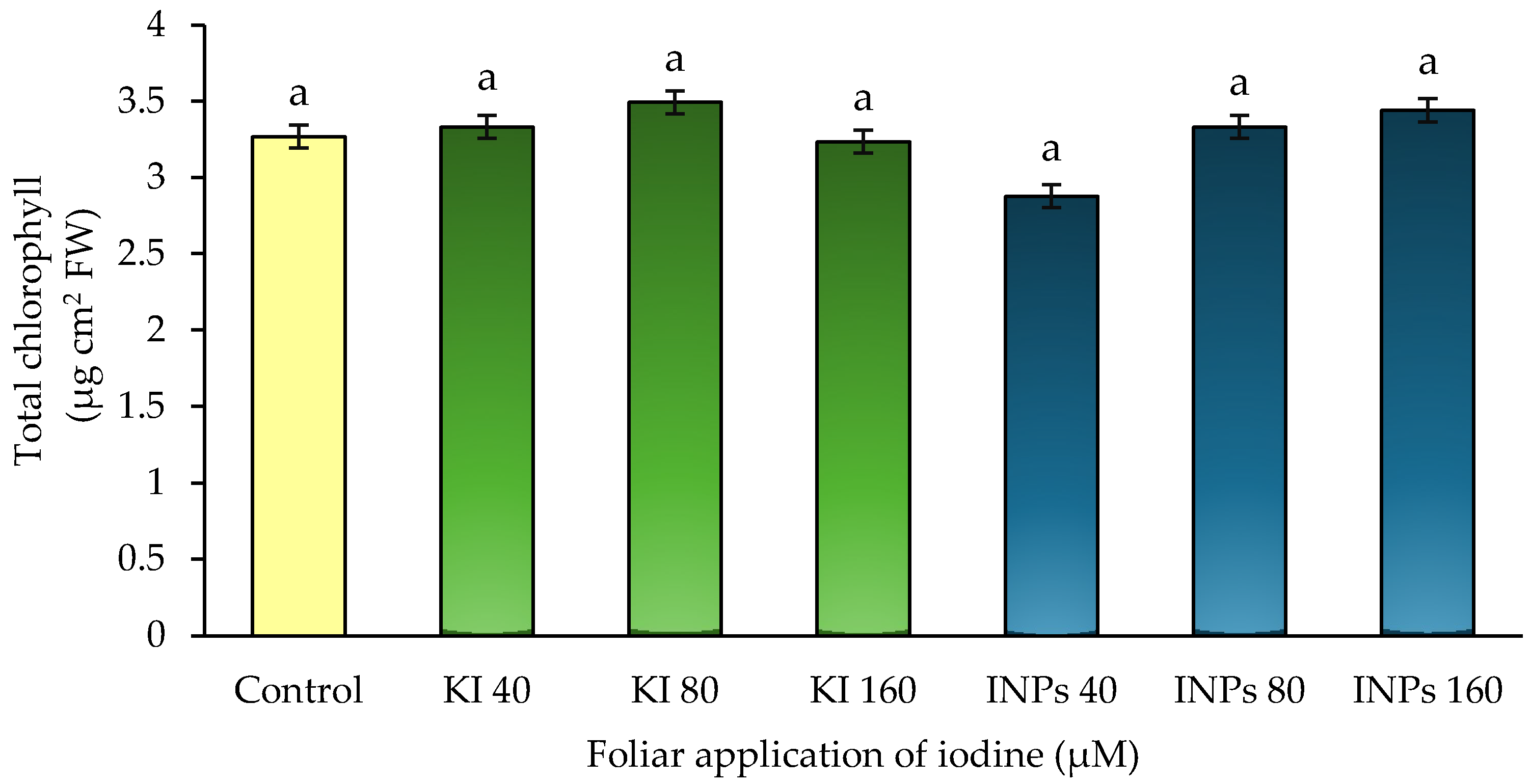
3.4. Total Chlorophyll
Total chlorophyll concentration is a key functional indicator of photosynthetic efficiency and is closely related to nitrogen metabolism, since its biosynthesis requires the incorporation of magnesium into the porphyrin ring, a process dependent on nitrogen, iron, and various enzymes related to the formation of tetrapyrroles [
39].
In this study, no statistically significant differences (
p > 0.05) were observed in total chlorophyll content between treatments (
Figure 6). However, different physiological trends were recorded depending on the chemical form of iodine applied. Foliar application of potassium iodide (KI) maintained chlorophyll levels similar to or slightly higher than the control, while treatments with iodine nanoparticles (INPs) at 40 and 80 µM showed moderate reductions in chlorophyll content, between 5% and 8%.
This behavior dependent on the chemical form is consistent with previous studies. Silva-Marrufo et al. [
8] reported that the application of KI in strawberries significantly increased the concentration of nitrogen in plant tissue and the content of photosynthetic pigments. Similarly, Shalaby [
18] observed that iodide treatments in lettuce favored amino acid accumulation, antioxidant defense, and photosynthetic apparatus stability. In spinach, Smoleń and Sady [
30] demonstrated that foliar application of iodide favors nitrogen metabolism and the maintenance of chlorophyll levels.
Our results reinforce this evidence by showing that KI maintains pigment integrity without inducing physiological stress. In contrast, INPs—although they supply iodine—could induce different physiological effects. Recent studies have reported that certain iodine formulations or nanoparticles can cause redox imbalances or modify antioxidant metabolism in plant tissues [
14]. Furthermore, it has been suggested that metal nanoparticles—including those containing iodine—have the potential to induce the generation of reactive oxygen species (ROS), which could negatively affect key enzymes in chlorophyll biosynthesis or alter the bioavailability of Mg
2+ [
40]. Although the specific role of INPs in this mechanism still requires further direct evidence, these processes could contribute to the observed reductions.
In addition, the subcellular compartmentalization of INPs in structures such as the apoplast or vacuoles, documented in other types of nanoparticles, could limit their direct interaction with the pigment biosynthetic machinery, unlike the efficient absorption of free iodide ions.
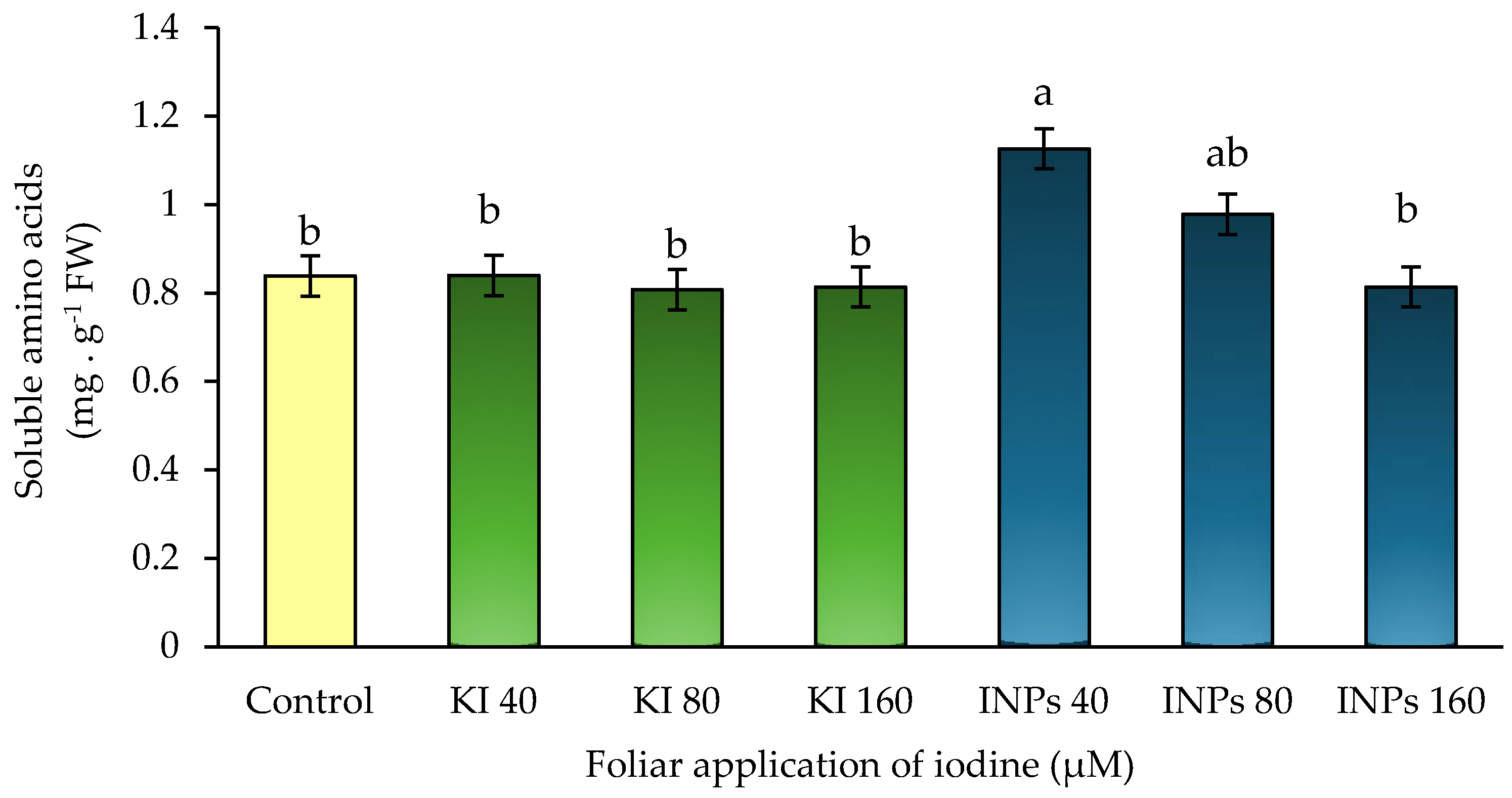
3.5. Concentration of Soluble Amino Acids
Soluble amino acids play central roles in nitrogen metabolism, acting not only as protein precursors but also as osmolyte buffers, redox buffers, and temporary reservoirs of reusable nitrogen, especially in response to environmental stimuli or fine metabolic adjustments. Their concentration reflects the balance between nitrogen assimilation, glutamate cycle enzyme activity, and intracellular redox signals [
41,
42].
In this study, statistically significant differences (
p ≤ 0.05) were detected in soluble amino acid levels (
Figure 7). Iodine nanoparticles (INPs) at 40 µM promoted a 30% increase compared to the control, while higher concentrations (80 and 160 µM) also tended to increase, although without exceeding the threshold of significance. Potassium iodide (KI), on the other hand, did not induce any relevant changes in this parameter.
This dose-dependent pattern of INPs suggests selective activation of nitrogen biosynthetic pathways without compromising cell integrity. Recent studies in lettuce have shown that certain forms of biofortifying iodine, in combination with micronutrients, can modulate the metabolism of nitrogen compounds, promoting assimilation efficiency and internal redistribution [
30].
Similarly, it has been documented that metal nanoparticles, such as zinc, iron, or selenium, can induce transient redox signaling responses, activating metabolic pathways associated with the accumulation of amino acids and antioxidant compounds, in the absence of overt toxicity [
35]. Although there are no specific studies on these effects in iodine nanoparticles, the results of this study suggest a potentially similar physiological behavior.
Given that all treatments were carried out under homogeneous nitrogen conditions (6 mM NH
4NO
3 in modified Hoagland solution), widely validated for
Lactuca sativa [
41], it can be inferred that the differences observed do not derive from nutritional limitation, but from a direct effect of the chemical form and concentration of iodine on primary metabolism.
The high response to 40 µM INPs could be related to greater absorption efficiency or progressive release of iodine at metabolically active sites, which would stimulate the synthesis or mobilization of amino acids from protein reserves or through reversible deamination. This type of internal reorganization has been documented in selenium- and iodine-based biofortification strategies, where an increase in low molecular weight nitrogen compounds is observed as part of a beneficial metabolic adjustment [
43].
3.6. Concentration of Soluble Proteins
Soluble proteins are fundamental components of nitrogen metabolism, as they comprise enzymes, transport proteins, cellular structures, and factors related to stress response. Their accumulation is directly associated with nitrogen availability, ammonium assimilation efficiency through the glutamine synthetase–glutamate synthetase (GS-GOGAT) cycle, and cellular redox balance [
3,
41,
42]. In addition, soluble protein content is recognized as a functional marker of nitrogen use efficiency, especially in leaf crops such as
Lactuca sativa [
30].
In the present study, statistically significant differences (
p ≤ 0.05) in soluble protein concentrations were observed between treatments (
Figure 8). The application of iodine nanoparticles (INPs) at 160 µM caused a significant decrease of approximately 20% compared to the control, while lower doses (40 and 80 µM) showed intermediate values with no significant differences. Potassium iodide (KI) treatments, at all concentrations evaluated (40, 80, and 160 µM), did not generate statistically relevant changes in soluble protein content.
This reduction associated with the highest dose of INPs suggests a physiological adjustment mechanism, in which high concentrations of iodine in nanoparticulate form could induce a metabolic change that favors the redistribution of nitrogen to alternative pathways or stress mitigation mechanisms, such as the biosynthesis of phenolic compounds or protein recycling [
8,
44]. Although no significant changes in amino acid levels were observed at this dose (160 µM), the decrease in proteins could be due to selective proteolysis processes or the inhibition of biosynthetic pathways under conditions of high iodine exposure.
In contrast, the increase in soluble amino acids recorded with INPs at 40 µM was not accompanied by an increase in proteins, suggesting that low doses stimulate the accumulation of nitrogen precursors without necessarily promoting protein synthesis. This divergence supports the hypothesis that different concentrations of INPs generate different metabolic responses: biosynthetic at low doses and possibly catabolic or inhibitory at high doses. Taken together, these results indicate that the accumulation of soluble proteins in lettuce leaves is sensitive to both the chemical form and concentration of the iodine applied. While KI showed no obvious physiological effects under the conditions evaluated, INPs generated dose-dependent effects capable of stimulating or inhibiting processes related to nitrogen metabolism. This underscores the importance of carefully adjusting iodine formulations and concentrations in biofortification strategies that seek to improve nitrogen efficiency without compromising physiological stability.
Similar trends have been documented in biofortification studies with selenium or zinc nanoparticles, in which high doses induce oxidative stress, while moderate concentrations improve stress tolerance and nitrogen-related metabolism [
45,
46,
47]. Our findings provide new evidence of a similar dual behavior in iodine nanoparticles, highlighting their complex interaction with protein turnover and nitrogen partitioning [
31].
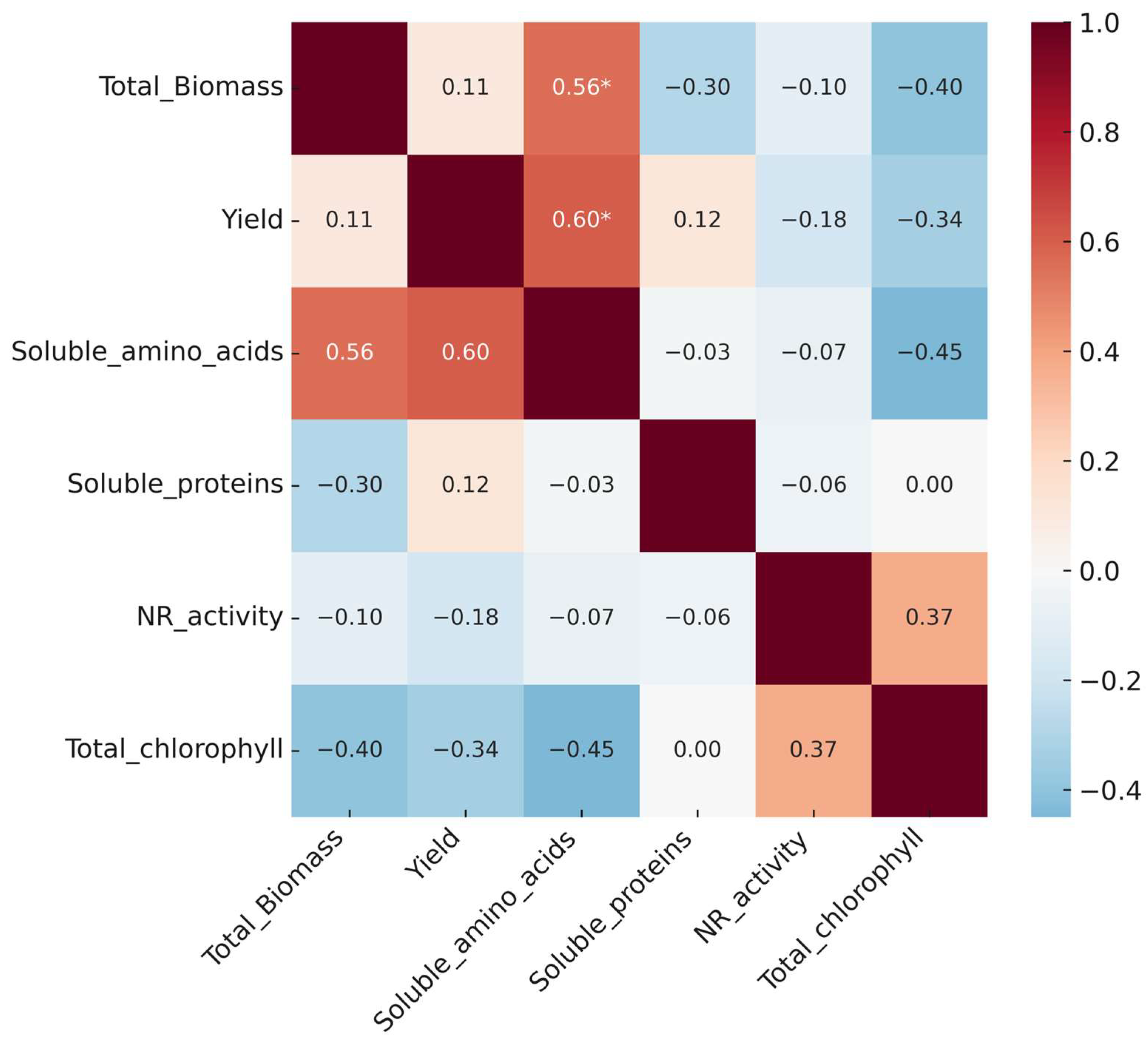
3.7. Heat Map and Correlation
Correlation analysis is a useful tool in plant physiology for identifying linear associations between biochemical and physiological variables that reflect nitrogen nutrition status, such as nitrogen assimilation, protein synthesis, photosynthetic activity, and growth [
48]. In this study, Pearson’s matrix (
Figure 9) showed positive and significant correlations (
p ≤ 0.05) between soluble amino acid content and both yield (r = 0.60 *) and total biomass (r = 0.56 *). This relationship is consistent with reports in which higher accumulation of free amino acids is associated with more efficient nitrogen use and improved productivity, without depending on a specific treatment [
49,
50].
Although these associations do not imply causality, they support the idea that high internal amino acid availability may reflect a more efficient nitrogen economy, a phenomenon observed in systems applying nanometric fertilizers—such as MoS
2 nanoparticles, which enhanced carbon and nitrogen assimilation and induced higher amino acid levels without immediate biomass gains [
51]. In contrast, soluble protein content and nitrate reductase (NR) activity did not show significant correlations with biomass or yield, suggesting decoupled or delayed biochemical responses, as documented in lettuce under different nitrogen application regimes [
52].
Moderate negative correlations were also observed between total chlorophyll and biomass (r = −0.40), as well as between chlorophyll and soluble amino acids (r = −0.45). This pattern could reflect a pigment dilution effect due to increased structural leaf mass, without functional loss of the photosynthetic apparatus, a situation previously described in vegetables that maintain chlorophyll levels within functional ranges despite variations in growth [
50]. Thus, the correlations found highlight the role of soluble amino acids as early indicators of nitrogen status and productivity, in an analytical framework that integrates all treatments and does not allow the effects to be attributed to a single source or form of application.
4. Conclusions
Foliar application of iodine in lettuce, both in ionic and nanoparticulate form, generated differentiated physiological responses associated with nitrogen metabolism. In particular, iodine nanoparticles (INPs) at 40 μM promoted a significant increase in biomass and soluble amino acid accumulation without compromising photosynthetic stability, suggesting a favorable metabolic adjustment at the biochemical level. Potassium iodide (KI) treatments showed a clear stimulation of nitrate reductase activity, indicating its ability to activate nitrogen assimilation pathways, although this did not translate into yield improvements under the conditions evaluated.
These complementary responses suggest that the chemical form and dose of iodine determine different mechanisms of action, possibly mediated by the redox state and intracellular distribution of the element. Taken together, these findings provide preliminary evidence on the functional value of iodine as a modulator of nitrogen metabolism and open up prospects for its integration into precision fertilization strategies based on physiological indicators.