Improving Soil Fertility and Forage Production Using Spruce Bark Biochar in an Eastern Newfoundland Podzolic Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production and Characterization
2.2. Experimental Set-Up and Design
2.3. Soil Sample Preparation for Analysis
2.4. Experiment Evaluations
2.4.1. Soil Analysis
2.4.2. Forage and Root Sampling and Their Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Characterization of Acid Podzolic Soil
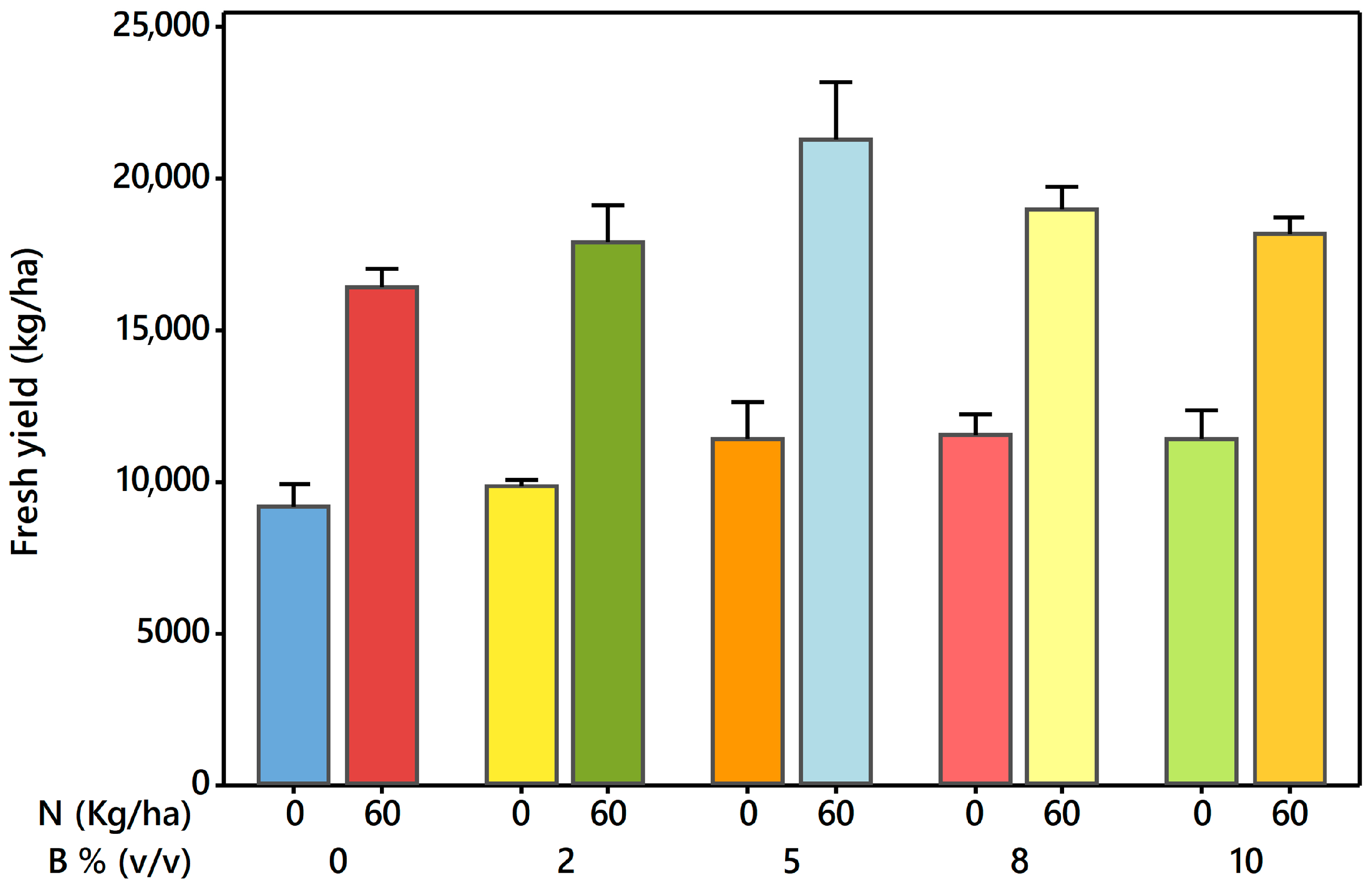
3.2. Treatment Effects on Forage Crop Yield
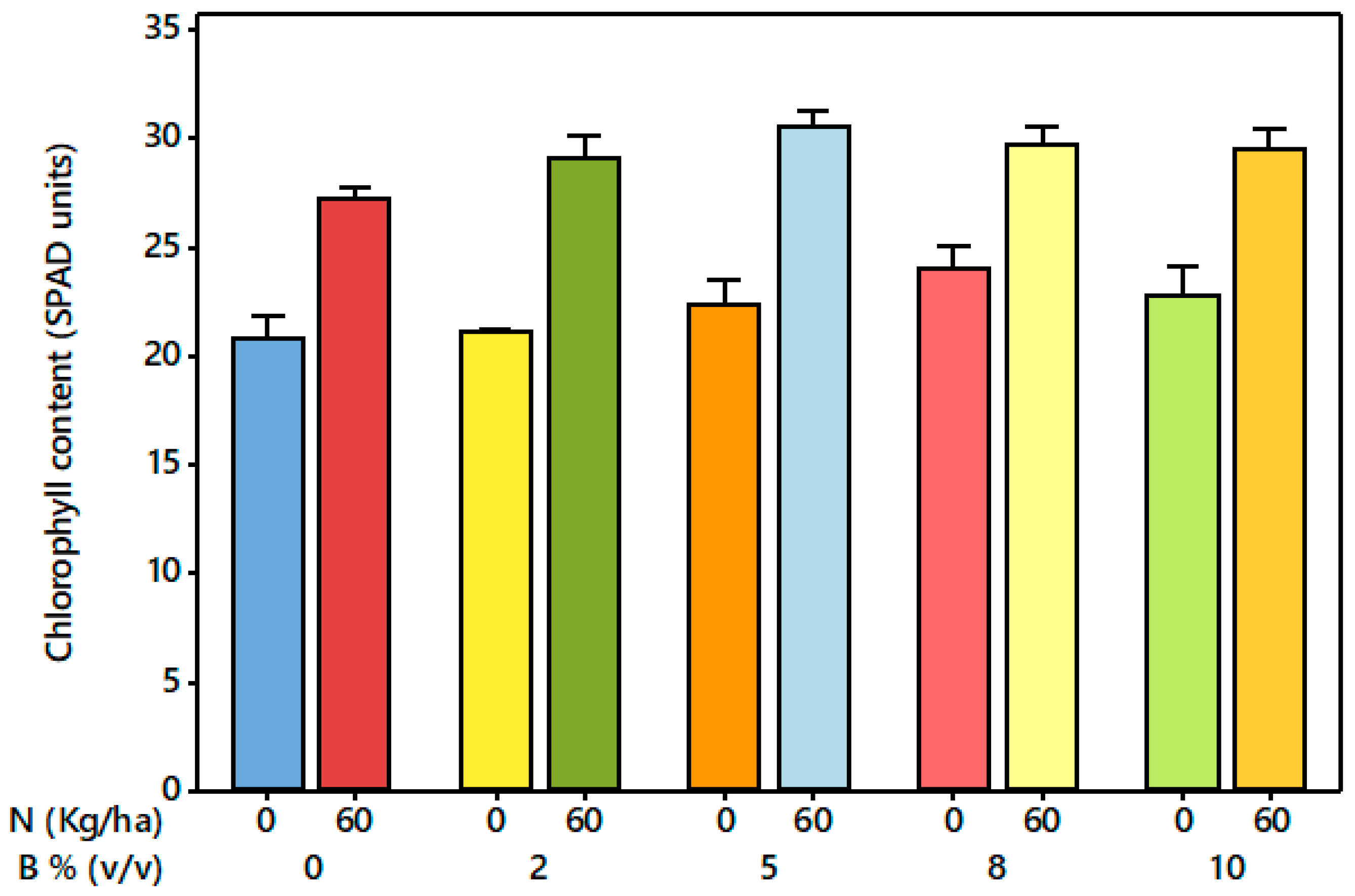
3.3. Treatment Effects on SPAD Value
3.4. Treatment Effects on Forage Quality
3.5. Treatment Effects on Forage Nutrient Uptake
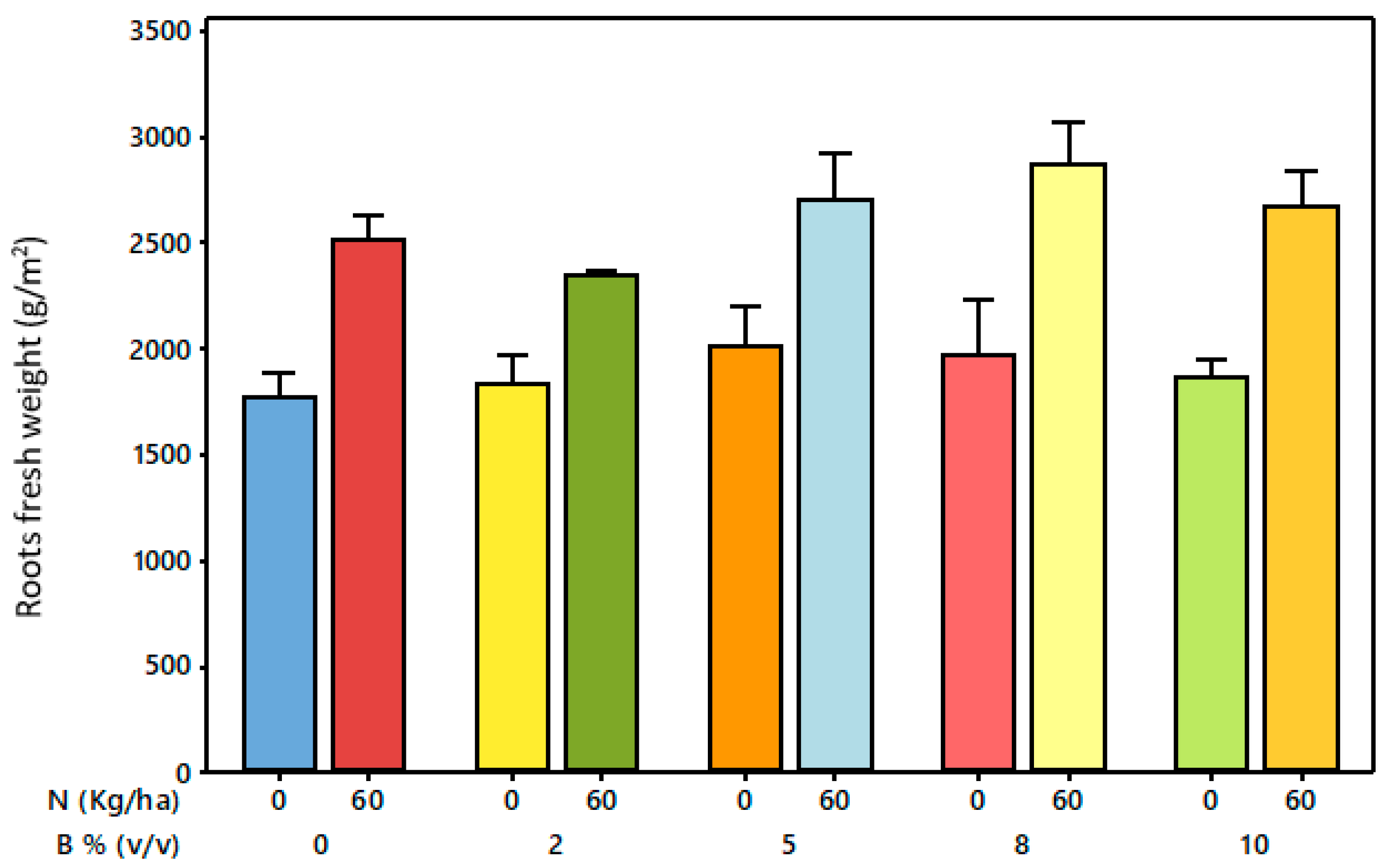
3.6. Treatment Effects on Forage Root
3.7. Treatment Effects on Root Nutrient Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Gunarathne, V.; Mayakaduwa, S.; Vithanage, M. Biochar’s influence as a soil amendment for essential plant nutrient uptake. In Essential Plant Nutrients; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–67. [Google Scholar]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 112, pp. 103–143. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems: A review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Yu, H.; Lu, K.; Harris, J.; Wang, X.; Hu, Z.; Cornelissen, G. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.D.; Johnson, R.L.; Lehmann, J.; Olk, D.C.; Neves, E.G.; Thompson, M.L. Abundant and stable char residues in soils: Implications for soil fertility and carbon sequestration. Environ. Sci. Technol. 2013, 46, 9571–9576. [Google Scholar] [CrossRef]
- Wan, Q.; Yuan, J.H.; Xu, R.K.; Li, X.H. Pyrolysis temperature influences ameliorating effects of biochars on acidic soil. Environ. Sci. Pollut. Res. 2014, 21, 2486–2495. [Google Scholar] [CrossRef]
- Abedin, J. Enhancing soils of Labrador through application of biochar, fishmeal, and chemical fertilizer. Soil Fertil. Crop Nutr. 2018, 110, 2576–2586. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B.; Boateng, A. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Lee, H.; Yang, J.E.; Ok, Y.S.; Skousen, J.; Kim, K.R. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Blackwell, P.; Abbott, L.K.; Storer, P. Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Aust. J. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Smolders, E.; Wang, H. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Koyama, S.; Katagiri, T.; Minamikawa, K.; Kato, M.; Hayashi, H. Effects of rice husk charcoal application on rice yield, methane emission, and soil carbon sequestration in andosol paddy soil. Jpn. Agric. Res. Q. 2016, 50, 319–327. [Google Scholar] [CrossRef]
- Zemanová, V.; Břendová, K.; Pavlíková, D.; Kubátová, P.; Tlustoš, P. Effect of biochar application on the content of nutrients (Ca, Fe, K, Mg, Na, P) and amino acids in subsequently growing spinach and mustard. Plant Soil Environ. 2017, 63, 322–327. [Google Scholar] [CrossRef]
- Eissa, R.; Jeyakumar, L.; McKenzie, D.B.; Wu, J. Influence of biochar feedstocks on nitrate adsorption capacity. Earth 2024, 5, 1080–1096. [Google Scholar] [CrossRef]
- Lange, S.F.; Allaire, S.E. Substrates Containing Biochar for White Spruce (Picea glauca sp.) Production in Nursery: Plant Growth, Economics, and Carbon Sequestration; Technical Report CRMR-2018-SA-2-EN; Centre de Recherche sur les Matériaux Renouvelables, Université Laval and GECA Environnement: Québec, QC, Canada, 2018. [Google Scholar]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Boller, B.; Posselt, U.K.; Veronesi, F. Fodder Crops and Amenity Grasses; Springer: New York, NY, USA, 2010. [Google Scholar]
- Heringa, P.K. Soils of the Avalon Peninsula, Newfoundland; Report No. 3; Research Branch, Agriculture Canada: St. John’s, NL, Canada, 1981. [Google Scholar]
- Adams, F.; Evans, C.E. A Rapid Method for Measuring Lime Requirement of Red-Yellow Podzolic Soils. Soil Sci. Soc. Am. J. 1962, 26, 355–357. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Chang, S.X.; Robison, D.J. Nondestructive and rapid estimation of hardwood foliar nitrogen status using the SPAD-502 chlorophyll meter. For. Ecol. Manag. 2003, 181, 331–338. [Google Scholar] [CrossRef]
- Minitab. Getting Started with Minitab 19 for Windows; Minitab, LLC.: State College, PA, USA, 2019. [Google Scholar]
- Dunn, P.K.; Smyth, G.K. Generalized Linear Models with Examples in R; Springer Nature: New York, NY, USA, 2018. [Google Scholar]
- Pirtle, T.; Rumble, L.; Klug, M.; Walker, F.; Cui, S.; Phillips, N. Impact of Biochar and Different Nitrogen Sources on Forage Radish Production in Middle Tennessee. J. Adv. Agric. 2019, 10, 1594–1610. [Google Scholar] [CrossRef]
- Singh, B.; Singh, Y.; Ladha, J.K.; Bronson, K.F.; Balasubramanian, V.; Singh, J.; Khind, C.S. Chlorophyll meter- and leaf color chart-based nitrogen management for rice and wheat in Northwestern India. Agron. J. 2002, 94, 821–829. [Google Scholar] [CrossRef]
- Sarfraz, R.; Shakoor, A.; Abdullah, M.; Arooj, A.; Xing, S. Impact of integrated application of biochar and nitrogen fertilizers on maize growth and nitrogen recovery in alkaline calcareous soil. Soil Sci. Plant Nutr. 2017, 63, 488–498. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, C.; Yang, L.; Huang, Y.; Zhou, L.; Wang, Y.; Pan, G. Biochar derived from corn straw affected availability and distribution of soil nutrients and cotton yield. PLoS ONE 2018, 13, e0190056. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Van Der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H.; et al. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: Options and mitigation strength in a global perspective. Glob. Change Biol. 2019, 655, 2077–2093. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A. Biochar as a soil amendment. Soil Sci. Annu. 2016, 67, 151–157. [Google Scholar] [CrossRef]
- Radulov, I.; Berbecea, A. Nutrient management for sustainable soil fertility. In Nutrient Management for Sustainable Soil Fertility; IntechOpen: London, UK, 2024. [Google Scholar]
- Oram, N.J.; van de Voorde, T.F.J.; Ouwehand, G.-J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Van Groenigen, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Elli, E.F.; Silva, D.D.; Silva, J.A.; Carvalho, F.S.; Santos, R.M.; Oliveira, P.T.; Lima, G.F.; Souza, M.A.; Ferreira, L.C.; Costa, H.R. Effects of growth reducer and nitrogen fertilization on morphological variables, SPAD index, interception of radiation and productivity of wheat. Afr. J. Agric. Res. 2015, 10, 577–582. [Google Scholar] [CrossRef]
- Muni, S.; Rout, K.K.; Parida, R.C. Effect of amendment of soil with fly ash in combination with other nutrients on change in grain yield, crude protein, NPN and true protein contents of rice. Ann. Agric. Res. 2016, 50, 378–381. [Google Scholar] [CrossRef]
- Revell, K.T.; Maguire, R.O.; Agblevor, F.A.; Sites, F. Field trials with poultry litter biochar and its effect on forages, green peppers, and soil properties. Commun. Soil Sci. Plant Anal. 2012, 43, 573–579. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal: A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Rees, F.; Sterckeman, T.; Morel, J.L. Root development of non-accumulating and hyperaccumulating plants in metal-contaminated soils amended with biochar. Plant Soil 2016, 142, 48–55. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Yang, Q.; Ravnskov, S.; Andersen, M.N. Nutrient uptake and growth of potato: Arbuscular mycorrhiza symbiosis interacts with quality and quantity of amended biochars. Plant Nutr. Soil Sci. 2020, 183, 220–232. [Google Scholar] [CrossRef]
- Coumaravel, K.; Santhi, R.; Maragatham, S. Effect of biochar on yield and nutrient uptake by hybrid maize and on soil fertility. Int. J. Agric. Sci. 2015, 49, 185–188. [Google Scholar] [CrossRef]
- Maftu, E.; Nursyamsi, D. Effect of biochar on peat soil fertility and NPK uptake by corn. J. Zhejiang Univ. B 2019, 41, 64–73. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, L.X.; Zhang, H.P.; Li, X.Y.; Shen, Y.F.; Li, S.Q. Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 2016, 67, 495–507. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. J. Plant Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Win, K.T.; Okazaki, K.; Ookawa, T.; Yokoyama, T.; Id, Y.O. Influence of rice-husk biochar and Bacillus pumilus strain TUAT-1 on yield, biomass production, and nutrient uptake in two forage rice genotypes. PLoS ONE 2019, 14, e0222090. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 2012, 41, 1033–1043. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Liu, S.; Xixian, H.; Zhongwu, L.; Xiaofei, T. Potential benefits of biochar in agricultural soils: A review. Pedosphere 2017, 27, 645–661. [Google Scholar] [CrossRef]
- Wang, J.; Tu, X.; Zhang, H.; Cui, J.; Ni, K.; Chen, J.; Yi, C.; Zhang, J.; Chang, S.X. Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci. Total Environ. 2020, 747, 141340. [Google Scholar] [CrossRef] [PubMed]
- Custos, J.M.; Moyne, C.; Sterckeman, T. How root nutrient uptake affects rhizosphere pH: A modelling study. Geoderma 2020, 369, 114314. [Google Scholar] [CrossRef]
- Kedir, A.J.; Zhang, M.; Unc, A. Understanding soil fertility status in Newfoundland from standard farm soil tests. Can. J. Soil Sci. 2021, 101, 517–531. [Google Scholar] [CrossRef]
| Analysis | SB550 |
|---|---|
| Moisture Content (%) | <1 |
| pH | 9.9 |
| Total Nitrogen, N (%) | 0.95 |
| Total Carbon, C (%) | 77.2 |
| Total Phosphorus, P (%) | 0.29 |
| Total Potassium, K (%) | 1.33 |
| Total Calcium, Ca (%) | 1.75 |
| Total Magnesium, Mg (%) | 0.24 |
| Total Iron, Fe (mg L−1) | 7690 |
| Total Copper, Cu (mg L−1) | 26 |
| Total Manganese, Mn (mg L−1) | 330 |
| Total Zinc, Zn (mg L−1) | 400 |
| Total Boron, B (mg L−1) | 64 |
| Total Sodium, Na (mg L−1) | 352 |
| Soluble Salts (dS m−1) | 0.7 |
| CEC (cmol kg−1) | 28.5 |
| Treatments | Sand (%) | Clay (%) | Silt (%) | Soil pH | N (%) | C (%) | SOM (%) | CEC (cmol kg−1) |
|---|---|---|---|---|---|---|---|---|
| Original Soil | 29.3 | 17.9 | 52.8 | 6.1 | 0.5 | 6.8 | 7.94 | 9.5 |
| Parameters | P (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) | S (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) | Na (mg kg−1) | Fe (mg kg−1) | B (mg kg−1) | Mn (mg kg−1) | Al (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original soil | 77 | 153 | 1305 | 300 | 23 | 3.1 | 3.7 | 38 | 140 | 1 | 16 | 1204 |
| Treatments | Soil pH | N (%) | C (%) | SOM (%) | CEC (cmol kg−1) |
|---|---|---|---|---|---|
| B0%-N0-C | 6.1 d | 0.5 | 6.70 r | 6.19 m | 17.4 ef |
| B2%-N0-C | 6.3 cd | 0.6 | 11.0 n | 8.58 a | 17.9 cd |
| B5%-N0-C | 6.3 cd | 0.6 | 16.9 i | 6.96 j | 17.1 fg |
| B8%-N0-C | 6.4 bcd | 0.6 | 16.0 j | 8.47 b | 17.4 ef |
| B10%-N0-C | 6.4 bcd | 0.6 | 18.5 f | 6.97 j | 18.3 ab |
| B0%-N60-C | 6.1 d | 0.5 | 7.10 q | 6.62 l | 17.6 de |
| B2%-N60-C | 6.2 d | 0.6 | 10.7 o | 7.49 g | 16.5 h |
| B5%-N60-C | 6.3 cd | 0.6 | 17.1 h | 7.95 e | 14.6 k |
| B8%-N60-C | 6.3 cd | 0.6 | 13.8 k | 6.87 k | 16.0 i |
| B10%-N60-C | 6.3 cd | 0.6 | 19.0 e | 8.03 d | 17.7 cde |
| B0%-N0-NC | 6.3 cd | 0.6 | 7.80 p | 7.66 f | 17.1 fg |
| B2%-N0-NC | 6.6 abc | 0.6 | 11.8 l | 8.47 b | 15.6 j |
| B5%-N0-NC | 6.7 ab | 0.6 | 18.2 g | 7.36 h | 18.0 bc |
| B8%-N0-NC | 6.9 a | 0.7 | 19.7 d | 7.23 i | 17.5 e |
| B10%-N0-NC | 6.8 a | 0.6 | 21.3 a | 6.97 j | 17.4 ef |
| B0%-N60-NC | 6.3 d | 0.6 | 7.80 p | 7.63 f | 17.9 cd |
| B2%-N60-NC | 6.6 abc | 0.6 | 11.3 m | 7.61 f | 18.6 a |
| B5%-N60-NC | 6.6 abc | 0.6 | 18.4 f | 8.36 c | 17.0 g |
| B8%-N60-NC | 6.8 a | 0.6 | 20.0 c | 6.95 j | 16.4 h |
| B10%-N60-NC | 6.8 a | 0.6 | 20.7 b | 6.67 l | 17.6 de |
| Treatments | P (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) | S (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) | Na (mg kg−1) | Fe (mg kg−1) | B (mg kg−1) | Mn (mg kg−1) | Al (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B0%-N0-C | 73.6 h | 18 o | 1426 t | 257 p | 22 cd | 2 kl | 3.5 k | 45 e | 171 b | 0.2 g | 54 i | 1258 d |
| B2%-N0-C | 104.6 a | 21 n | 1745 n | 313 k | 25 b | 2.6 j | 4 hi | 47 d | 161 f | 0.3 fg | 84 a | 1047 o |
| B5%-N0-C | 104.6 a | 54 k | 1879 i | 316 ij | 26 ab | 4 f | 3.9 i | 45 e | 160 fg | 0.5 de | 60 f | 1170 j |
| B8%-N0-C | 101.6 b | 65 i | 1827 j | 304 l | 25 b | 4 f | 3.7 j | 42 f | 154 i | 0.5 de | 57 g | 1172 i |
| B10%-N0-C | 100.6 b | 92 f | 1953 f | 318 i | 27 a | 4.6 d | 3.9 i | 41 f | 161 f | 0.6 cd | 47 j | 1165 k |
| B0%-N60-C | 88.6 f | 20 n | 1594 s | 275 o | 23 c | 2.7 j | 4.1 gh | 49 c | 166 cd | 0.2 g | 67 e | 1287 c |
| B2%-N60-C | 101.6 b | 21 n | 1772 l | 312 k | 25 abc | 2.6 j | 4.5 cd | 51 ab | 160 fg | 0.3 fg | 80 b | 1115 q |
| B5%-N60-C | 104.6 a | 29 m | 1760 m | 286 m | 23 c | 3.6 h | 4 hi | 50 bc | 159 g | 0.4 ef | 67 e | 1124 p |
| B8%-N60-C | 94 e | 30 m | 1738 o | 281 n | 25 b | 3.5 h | 3.7 j | 52 a | 155 i | 0.4 ef | 67 e | 1190 g |
| B10%-N60-C | 104.6 a | 43 l | 1759 n | 281 n | 22 cd | 3.9 g | 3.6 jk | 45 e | 157 h | 0.4 ef | 71 d | 1048 o |
| B0%-N0-NC | 98.6 c | 62 j | 1669 r | 353 g | 16 h | 2.1 k | 4.2 fg | 36 h | 174 a | 0.6 cd | 59 f | 1289 b |
| B2%-N0-NC | 93.6 e | 76 h | 1797 k | 386 c | 21 de | 2.9 i | 4.3 ef | 34 i | 165 de | 0.4 ef | 41 k | 1105 n |
| B5%-N0-NC | 93.6 e | 176 d | 2019 d | 405 b | 20 ef | 4.3 e | 4.6 bc | 36 h | 159 g | 0.7 bc | 33 n | 1163 l |
| B8%-N0-NC | 97.6 c | 251 a | 2050 c | 387 c | 21 cd | 5.4 a | 4.7 b | 39 g | 167 c | 0.8 b | 56 gh | 1135 o |
| B10%-N0-NC | 89.6 f | 233 b | 2057 b | 386 c | 18 g | 5.1 b | 4.7 b | 38 g | 174 a | 0.8 b | 73 c | 1193 f |
| B0%-N60-NC | 98.6 c | 64 i | 1690 p | 338 h | 20 ef | 1.9 l | 4.1 gh | 34 i | 173 a | 0.3 fg | 37 m | 1336 a |
| B2%-N60-NC | 86.6 g | 84 g | 2117 a | 450 a | 20 ef | 3.0 i | 5.1 a | 38 g | 166 cd | 0.6 cd | 39 l | 1217 e |
| B5%-N60-NC | 93.6 e | 145 e | 1892 h | 380 d | 19 fg | 3.8 g | 4.4 de | 34 i | 164 e | 0.6 cd | 32 n | 1140 n |
| B8%-N60-NC | 95.6 d | 205 c | 1916 g | 360 f | 22 cd | 4.9 c | 4.3 ef | 35 hi | 167 c | 0.8 b | 42 k | 1157 m |
| B10%-N60-NC | 88.6 f | 206 c | 1979 e | 371 e | 20 ef | 4.8 c | 4.3 ef | 39 g | 173 a | 1.5 a | 55 hi | 1184 h |
| Treatments | Crude Protein (%) | ADF (%) | NDF (%) | Est. TDN (%) | Dig. Energy |
|---|---|---|---|---|---|
| (Mcal kg−1) | |||||
| B0%-N0 | 5.16 | 21.80 | 37.20 | 76.56 | 3.37 |
| B2%-N0 | 5.40 | 20.40 | 34.76 | 78.40 | 3.44 |
| B5%-N0 | 5.50 | 20.23 | 34.76 | 78.60 | 3.46 |
| B8%-N0 | 5.63 | 19.03 | 33.33 | 79.83 | 3.52 |
| B10%-N0 | 6.00 | 19.30 | 34.06 | 80.20 | 3.51 |
| B0%-N60 | 5.26 | 20.73 | 37.50 | 77.86 | 3.43 |
| B2%-N60 | 5.40 | 20.20 | 35.00 | 78.66 | 3.45 |
| B5%-N60 | 5.70 | 19.93 | 35.13 | 79.03 | 3.46 |
| B8%-N60 | 5.76 | 19.40 | 34.36 | 79.70 | 3.50 |
| B10%-N60 | 6.13 | 19.33 | 34.26 | 79.80 | 3.51 |
| Treatments | N (kg ha−1) | P (kg ha−1) | K (kg ha−1) | Ca (kg ha−1) | Mg (kg ha−1) | Na (kg ha−1) | Fe (kg ha−1) | Cu (kg ha−1) | Mn (kg ha−1) | Zn (kg ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| B0%-N0 | 3.50 | 0.94 | 7.81 bc | 1.79 ef | 0.86 de | 0.80 bc | 110.3 | 8.76 | 255.6 | 12.00 |
| B2%-N0 | 3.62 | 0.96 | 9.82 a | 1.88 def | 0.98 cd | 0.52 bc | 147.6 | 8.23 | 174.3 | 11.00 |
| B5%-N0 | 3.61 | 0.94 | 11.17 a | 1.92 de | 0.91 de | 0.20 c | 116.0 | 8.43 | 149.6 | 11.66 |
| B8%-N0 | 3.66 | 1.04 | 11.13 a | 2.09 cd | 0.89 de | 0.13 c | 85.66 | 11.33 | 112.0 | 13.00 |
| B10%-N0 | 4.01 | 1.08 | 11.08 a | 2.43 b | 1.26 b | 0.14 c | 244.3 | 9.73 | 127.3 | 12.00 |
| B0%-N60 | 3.67 | 0.75 | 4.38 d | 1.60 f | 0.78 e | 2.57 a | 60.66 | 6.90 | 211.3 | 13.66 |
| B2%-N60 | 3.63 | 0.79 | 6.07 cd | 1.72 ef | 0.84 de | 1.62 ab | 44.66 | 6.60 | 161.3 | 10.66 |
| B5%-N60 | 3.84 | 0.78 | 9.81 a | 1.88 def | 0.94 de | 0.39 c | 41.00 | 6.16 | 85.33 | 15.00 |
| B8%-N60 | 3.94 | 0.88 | 9.63 ab | 2.28 bc | 1.14 bc | 0.27 c | 49.66 | 6.86 | 132.6 | 11.00 |
| B10%-N60 | 4.27 | 0.98 | 10.42 a | 2.90 a | 1.46 a | 0.16 c | 60.00 | 7.16 | 85.00 | 10.50 |
| Treatments | N (kg ha−1) | P (kg ha−1) | K (kg ha−1) | Ca (kg ha−1) | Mg (kg ha−1) | Na (kg ha−1) | Fe (kg ha−1) | Cu (kg ha−1) | Mn (kg ha−1) | Zn (kg ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| B0%-N0 | 13.02 | 4.23 | 14.80 | 8.99 | 1.79 | 2.35 | 1340.0 | 46.66 | 195.6 | 26.66 |
| B2%-N0 | 13.02 | 4.20 | 16.83 | 10.37 | 1.83 | 2.13 | 1303.3 | 35.33 | 124.0 | 19.66 |
| B5%-N0 | 14.08 | 4.95 | 25.02 | 10.11 | 2.11 | 1.90 | 1273.3 | 36.66 | 102.0 | 23.33 |
| B8%-N0 | 15.16 | 5.26 | 26.76 | 11.43 | 2.24 | 1.65 | 1486.6 | 41.66 | 134.3 | 27.66 |
| B10%-N0 | 16.41 | 6.13 | 30.78 | 12.65 | 2.36 | 1.77 | 1450.0 | 36.00 | 103.3 | 23.00 |
| B0%-N60 | 15.35 | 5.04 | 17.73 | 14.01 | 1.52 | 2.75 | 1406.6 | 51.66 | 122.3 | 23.00 |
| B2%-N60 | 16.13 | 4.75 | 20.24 | 14.41 | 1.81 | 3.25 | 1433.3 | 47.66 | 106.6 | 21.00 |
| B5%-N60 | 17.15 | 4.78 | 26.15 | 14.62 | 2.42 | 2.71 | 1366.6 | 59.33 | 89.33 | 25.33 |
| B8%-N60 | 19.68 | 5.67 | 29.60 | 16.53 | 3.09 | 2.04 | 1168.0 | 63.33 | 91.33 | 26.33 |
| B10%-N60 | 20.75 | 5.92 | 31.74 | 16.67 | 3.26 | 10.47 | 1049.6 | 55.66 | 62.33 | 24.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 His Majesty the King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, R.O.; Jeyakumar, L.; McKenzie, D.B.; Wu, J. Improving Soil Fertility and Forage Production Using Spruce Bark Biochar in an Eastern Newfoundland Podzolic Soil. Nitrogen 2025, 6, 83. https://doi.org/10.3390/nitrogen6030083
Eissa RO, Jeyakumar L, McKenzie DB, Wu J. Improving Soil Fertility and Forage Production Using Spruce Bark Biochar in an Eastern Newfoundland Podzolic Soil. Nitrogen. 2025; 6(3):83. https://doi.org/10.3390/nitrogen6030083
Chicago/Turabian StyleEissa, Riad O., Lordwin Jeyakumar, David B. McKenzie, and Jianghua Wu. 2025. "Improving Soil Fertility and Forage Production Using Spruce Bark Biochar in an Eastern Newfoundland Podzolic Soil" Nitrogen 6, no. 3: 83. https://doi.org/10.3390/nitrogen6030083
APA StyleEissa, R. O., Jeyakumar, L., McKenzie, D. B., & Wu, J. (2025). Improving Soil Fertility and Forage Production Using Spruce Bark Biochar in an Eastern Newfoundland Podzolic Soil. Nitrogen, 6(3), 83. https://doi.org/10.3390/nitrogen6030083






