Comparative Effects of Iron Nanoparticles, Chelates, and Iron Sulfate on Biomass, Yield, and Nitrogen Assimilation in Spinach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Crop Management
2.2. Characterization of Iron Oxide Nanoparticles IONPs
2.3. Nanoparticles Preparation
2.4. Experimental Design and Treatments
2.5. Plant Sampling
2.6. Plant Analysis
2.6.1. Biomass and Yield
2.6.2. Analysis of Photosynthetic Pigments
2.6.3. Chlorophyll Index
2.6.4. Extraction and Assay of NR
2.6.5. Determination of Soluble Amino Acids
2.6.6. Determination of Soluble Proteins
2.7. Statistical Analysis
3. Results and Discussion
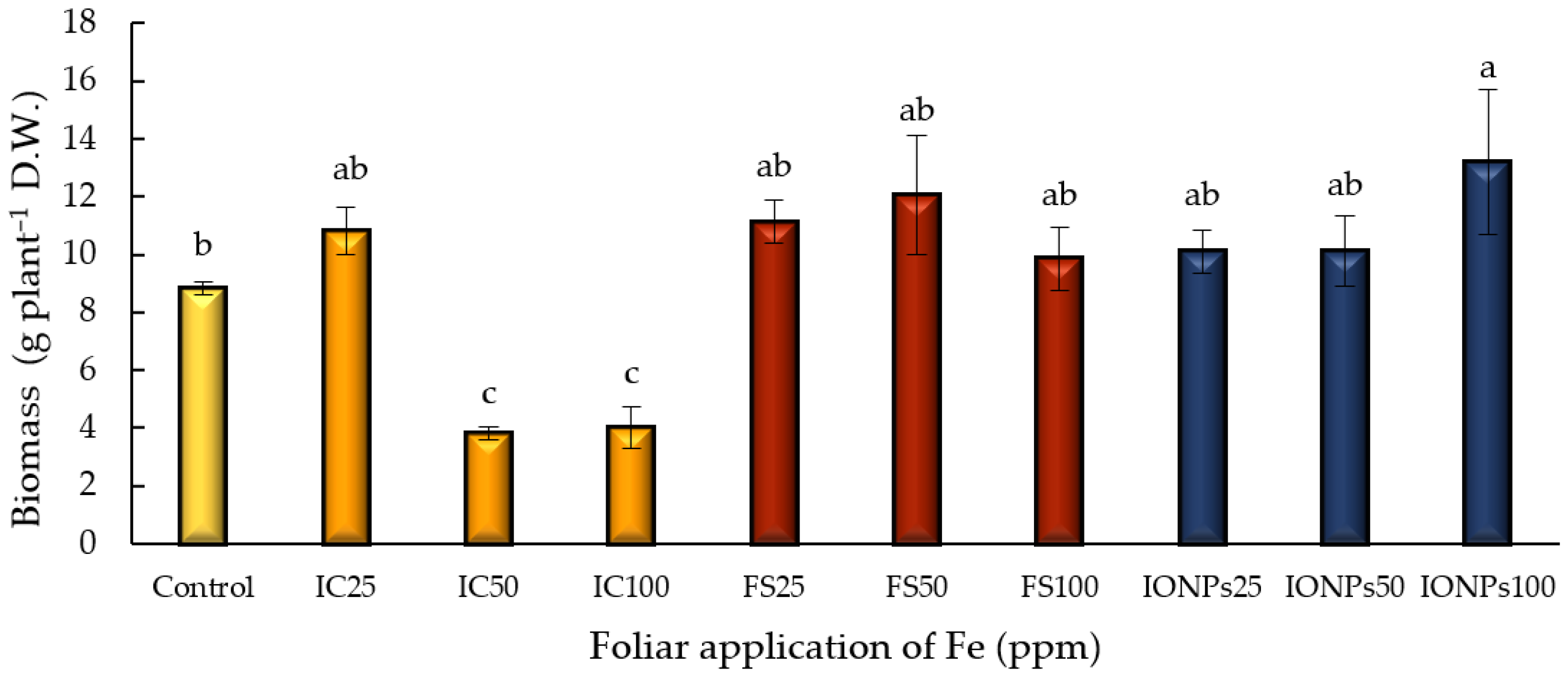
3.1. Biomass
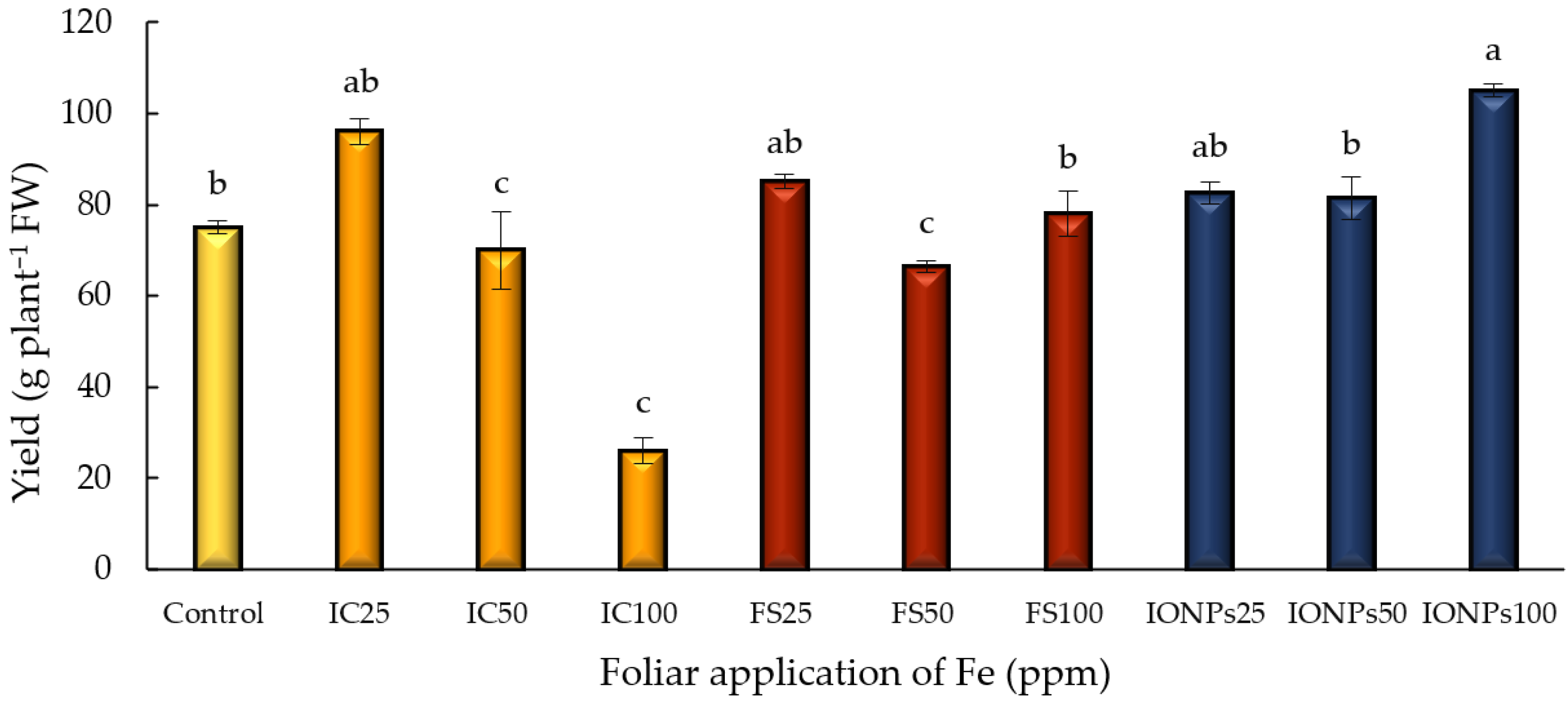
3.2. Yield
3.3. Photosynthetic Pigments
3.4. SPAD Values
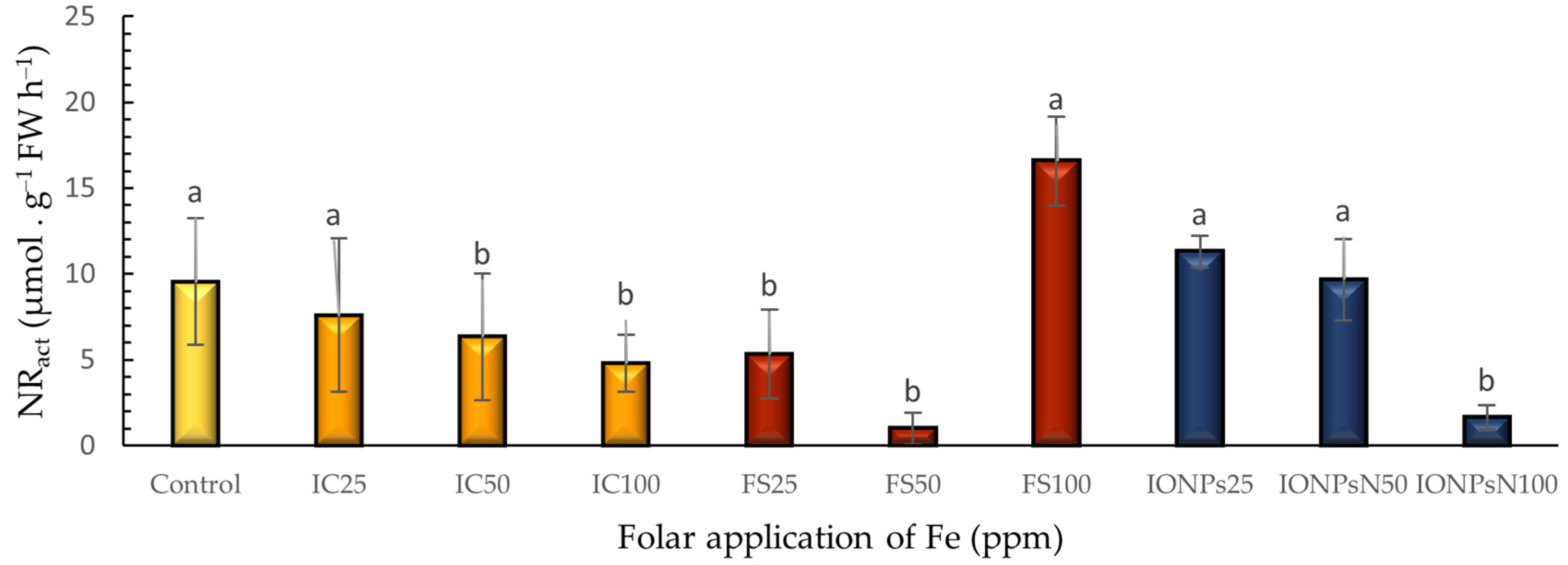
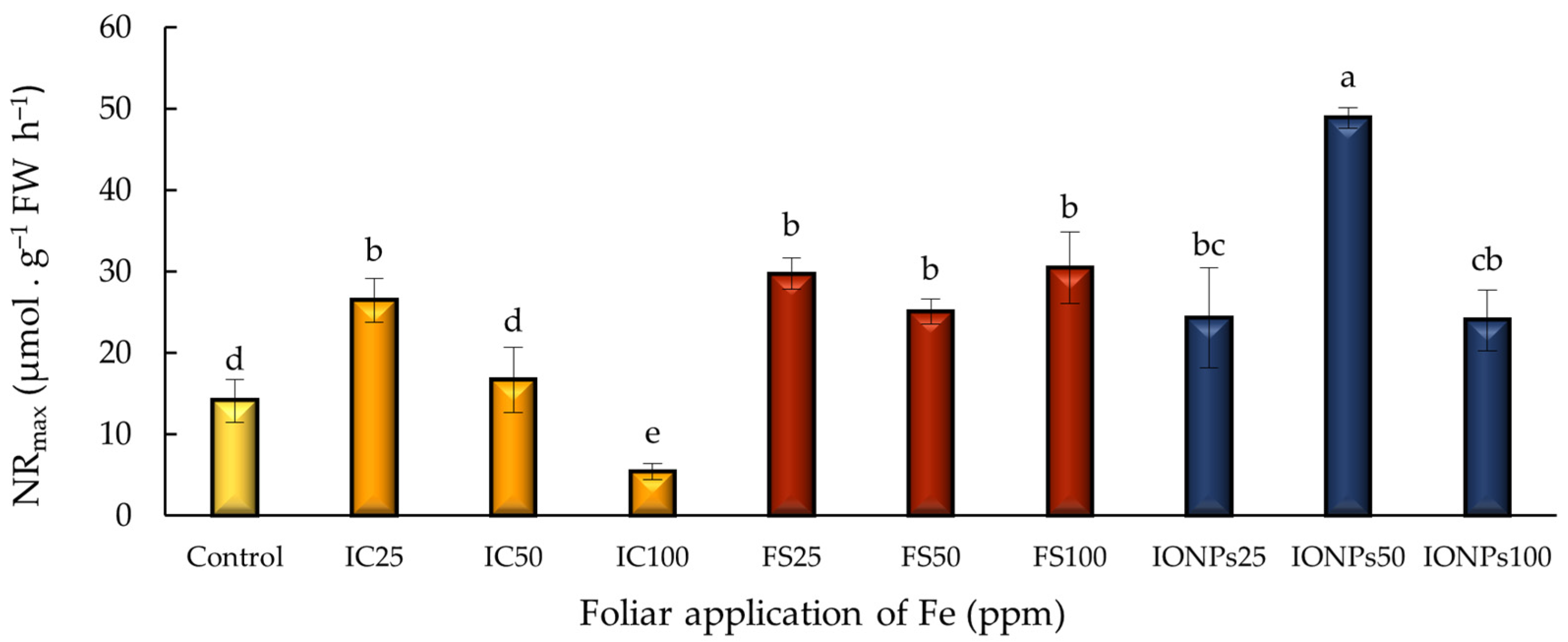
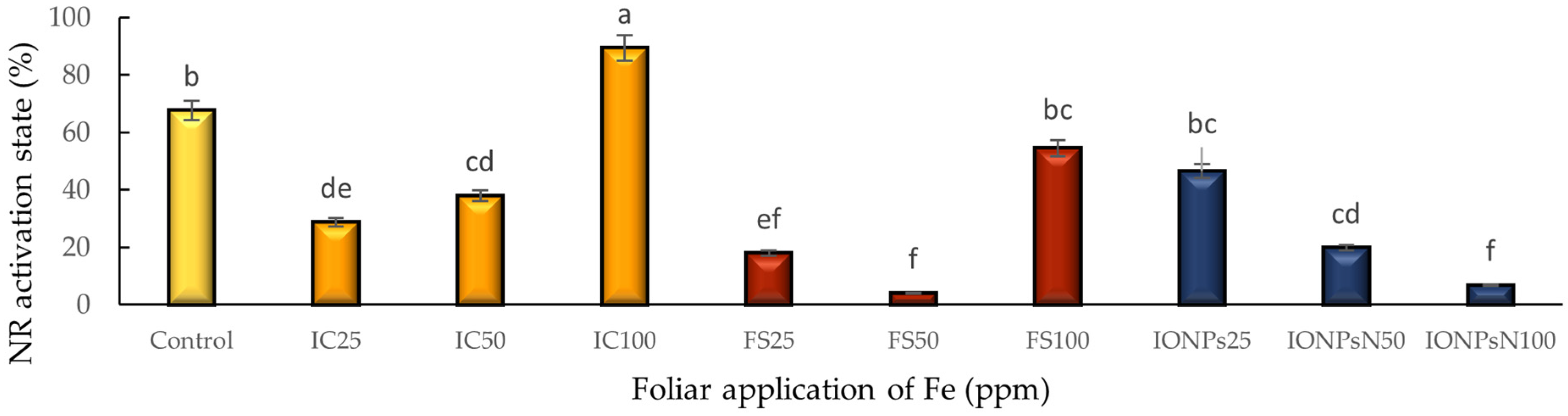
3.5. Nitrate Reductase Activity
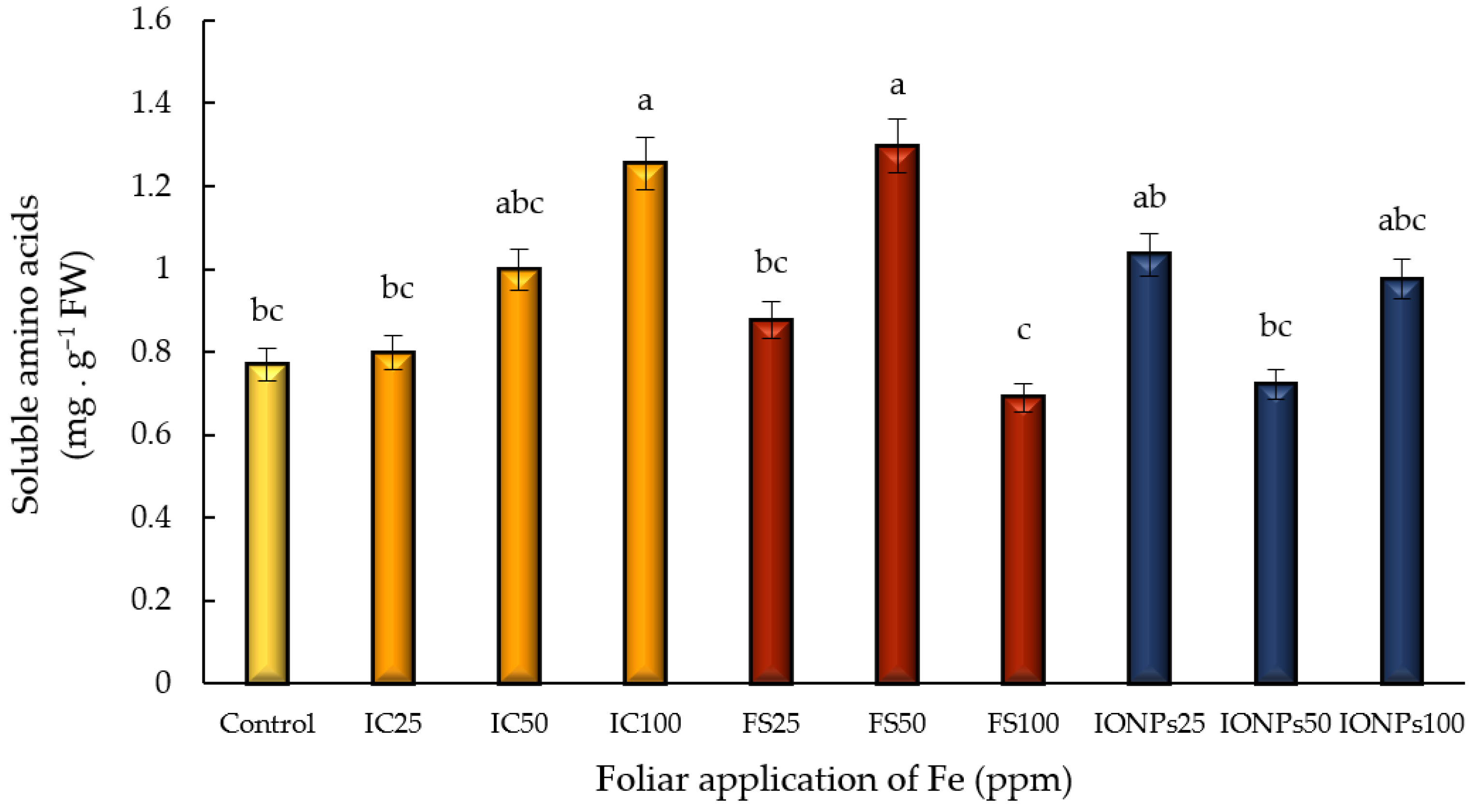
3.6. Soluble Amino Acids
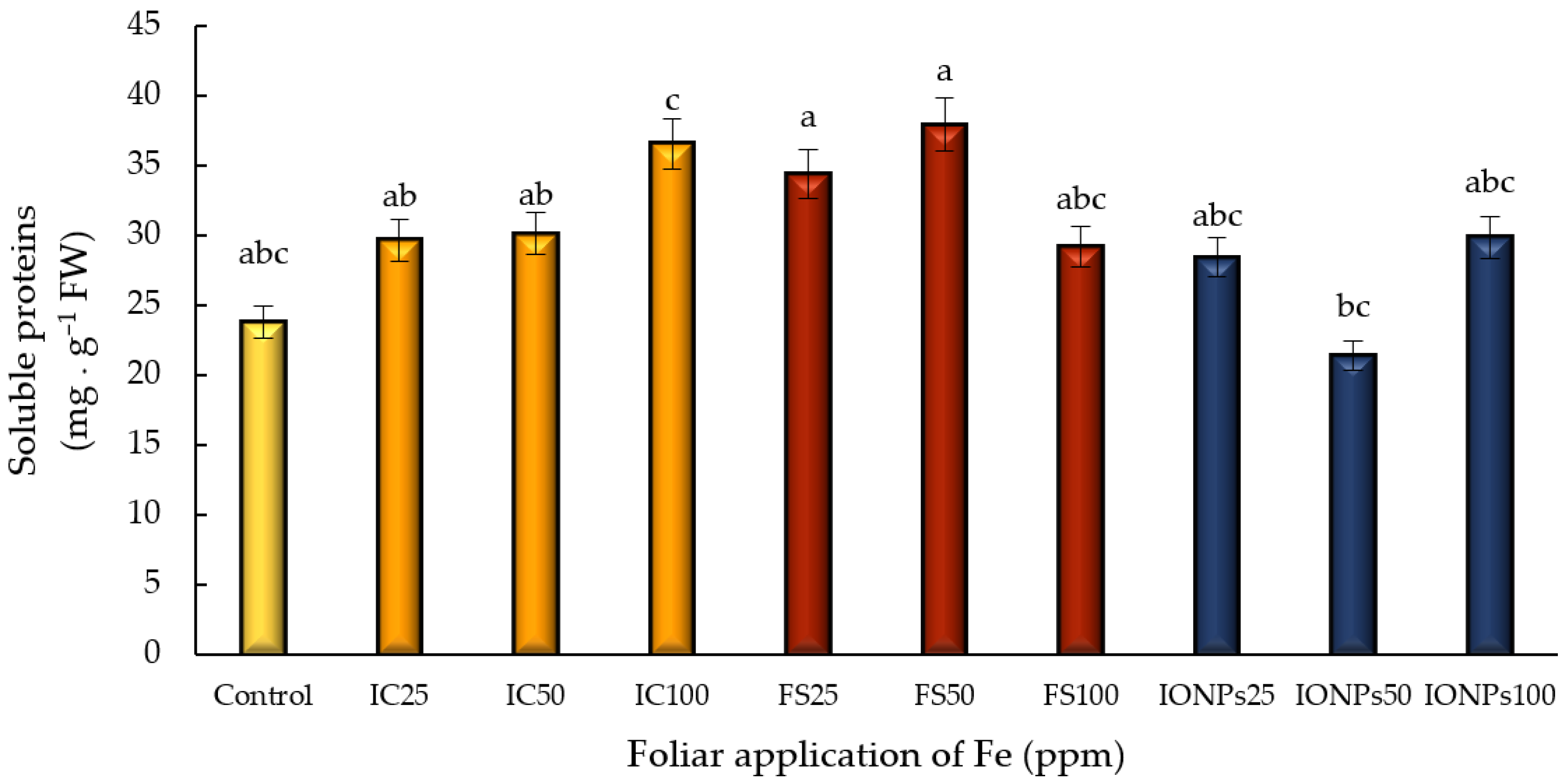
3.7. Soluble Proteins
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Zhang, F. Enhanced nitrogen deposition over China. Nature 2010, 494, 459–462. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Broadley, M.R.; Brown, P.R.; Cakmak, I.; Rengel, Z.; Zhao, F.J. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 191–248. [Google Scholar]
- Briat, J.F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, noxious, and not readily available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.M.; Mohamed, M.H.; Sadak, M.S.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits, and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 2021, 45, 177. [Google Scholar] [CrossRef]
- Mondal, A.; Paul, S.; Pal, R.; Paul, S. Nano iron loaded algal biomass: For better yield, amino acid, and iron content in rice—A ‘nano-phycofertilizer’. Algal Res. 2024, 81, 103573. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Shafiei-Masouleh, S.-S.; Mohsin, R.M.; Salih, Z.K. Foliar application of iron oxide nanoparticles promotes growth, mineral contents, and medicinal qualities of Solidago virgaurea L. J. Soil Sci. Plant Nutr. 2023, 23, 2610–2624. [Google Scholar]
- Guillén-Enríquez, R.R.; Zuñiga-Estrada, L.; Ojeda-Barrios, D.L.; Rivas-García, T.; Trejo-Valencia, R.; Preciado-Rangel, P. Effect of nano-biofortification with iron on yield and bioactive compounds in cucumber. Rev. Mex. Cienc. Agríc. 2022, 13, 173–184. [Google Scholar]
- Gutiérrez-Ruelas, N.J.; Palacio-Márquez, A.; Sánchez, E.; Muñoz-Márquez, E.; Chávez-Mendoza, C.; Ojeda-Barrios, D.L.; Flores-Córdova, M.A. Impact of the foliar application of nanoparticles, sulfate and iron chelate on the growth, yield and nitrogen assimilation in green beans. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12437. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Schwab, A.P. The chemistry of iron in soils and its availability to plants. J. Plant Nutr. 1982, 5, 821–840. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Koso-bryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Na-noparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Zhou, Z.; Tao, Y.; Zhang, Y.; Mu, Y.; Wu, S.; Nie, L. Foliar Application of Zinc Oxide Nanoparticles Improved Yield and 2-Acetyl-1-pyrroline Content in Fragrant Rice under Salt Stress. Crop Environ. 2025, 4, 107–117. [Google Scholar] [CrossRef]
- Murcia, M.A.; Jiménez-Monreal, A.M.; González, J.; Martínez-Tomé, M. Spinach (Spinacia oleracea L.). In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: London, UK, 2020; pp. 181–195. [Google Scholar]
- Santamaria, P.; Campanile, G.; Parente, A.; Elia, A. Subirrigation vs drip-irrigation: Effects on yield and quality of soilless grown cherry tomato. J. Hortic. Sci. Biotechnol. 2003, 78, 290–296. [Google Scholar] [CrossRef]
- Sánchez, E.; Ruiz, J.M.; Romero, L. Proline metabolism in response to nitrogen toxicity in fruit of French Bean plants (Phaseolus vulgaris L. cv Strike). Sci. Hortic. 2002, 93, 225–233. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T. Seed nano-priming with zinc oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef]
- Schucknecht, A.; Hammerle, A.; Wohlfahrt, G.; Tappeiner, U.; Wieser, G. Estimating dry biomass and plant nitrogen concentration in pre-Alpine grassland vegetation. Biogeosciences 2022, 19, 2699–2717. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Shrestha, S.; Brueck, H.; Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B Biol. 2012, 113, 7–13. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Brendle-Behnisch, E. Acid-base-modulation of nitrate reductase in leaf tissues. Planta 1995, 196, 1–6. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 15.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Singh, S.P.; Dutta, S.K.; Jha, S.; Prasad, S.S. Nutrient management in calcareous soil improves rice–maize sustainable yield index, performance indicators. Commun. Soil Sci. Plant Anal. 2021, 52, 701–716. [Google Scholar] [CrossRef]
- Dos Santos, V.R.; Costa, L.C.; Rocha, A.M.S. Biomass accumulation, extraction and nutrient use efficiency by cover crops. Res. Soc. Dev. 2020, 9, e9433108811. [Google Scholar] [CrossRef]
- Yousaf, N.; Ishfaq, M.; Qureshi, H.A.; Saleem, A.; Yang, H. Characterization of root and foliar-applied iron oxide nanoparticles (α-Fe2O3, γ-Fe2O3, Fe3O4, and bulk Fe3O4) in improving maize (Zea mays L.) growth and physiology. Nanomaterials 2023, 13, 3036. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yue, L.; Wang, C.; Luo, X.; Zhang, C.; Zhao, X.; Wu, F. Foliar application with iron oxide nanomaterials stimulate nitrogen fixation, yield, and nutritional quality of soybean. ACS Nano 2022, 16, 8500–8514. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Ayad, A.A.; Abdel-Aziz, H.S.M. Foliar application of different iron sources improves morpho-physiological traits and nutritional quality of broad bean grown in sandy soil. Plants 2022, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Tombuloglu, G.; Tombuloglu, H.; Slimani, Y. Effects of foliar iron oxide nanoparticles (Fe3O4) application on photosynthetic parameters, distribution of mineral elements, magnetic behaviour, and photosynthetic efficiency. Eur. J. Agron. 2024, 149, 126849. [Google Scholar]
- Nasar, J.; Wang, G.-Y.; Zhou, F.-J.; Gitari, H.; Zhou, X.-B.; Tabl, K.M.; Hasan, M.E.; Ali, H.; Waqas, M.M.; Ali, I.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Drostkar, E.; Talebi, R.; Kanouni, H. Foliar application of Fe, Zn and NPK nano-fertilizers on seed yield and morphological traits in chickpea under rainfed condition. J. Resour. Ecol. 2016, 4, 221–228. [Google Scholar]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhu, S. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Eur. J. Agron. 2022, 135, 126492. [Google Scholar] [CrossRef] [PubMed]
- Kroh, G.E.; Pilon, M. Regulation of iron homeostasis and use in chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Saito, A. Elucidation of efficient photosynthesis in plants with limited iron. Soil Sci. Plant Nutr. 2022, 68, 556–567. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, N.N.; Pan, Q.; Lin, X.Y.; Shangguan, Z. Hydrogen sulphide alleviates iron deficiency by promoting iron availability and plant hormone levels in Glycine max seedlings. BMC Plant Biol. 2020, 20, 467. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R. Nanotechnology Applied in Agriculture: Controlled Release of Agrochemicals. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer: Cham, Switzerland, 2015; pp. 103–118. [Google Scholar]
- Yaneva, I.A.; Baydanova, V.D.; Vunkova Radeva, R.V. Nitrate reductase activation state in leaves of molybdenum deficient winter wheat. J. Plant Physiol. 2000, 157, 495–501. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar]
- Tejada-Jimenez, M.; Llamas, A.; Galván, A.; Fernández, E. Role of Nitrate Reductase in NO Production in Photosynthetic Eukaryotes. Plants 2019, 8, 56. [Google Scholar] [CrossRef]
- Gohar, F.; Iqbal, U.; Khan, M.B.; Rehman, F.; Maryam, F.; Azmat, M.; Munir, S.; Shahid, M. Impact of Nanoparticles on Plant Growth, Development and Physiolo-gical Processes: A Comprehensive Review. SM J. Med. Plant Stud. 2025, in press.
- Correia, M.J.; Fonseca, F.; Azedo-Silva, J.; Dias, C.; David, M.M.; Barrote, I.; Osório, M.L.; Osório, J. Effects of water deficits on the activity of nitrate reductase and content of sugars, nitrate and free amino acids in the leaves and roots of sunflower and white lupin plants growing under two nutrient supply regimes. Physiol. Plant. 2005, 123, 190–203. [Google Scholar] [CrossRef]
- Borlotti, A.; Vigani, G.; Zocchi, G. Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants. BMC Plant Biol. 2012, 12, 189. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Huber, S.C. Post-translational regulation of nitrate reductase: Mechanism, physiological relevance and environmental triggers. J. Exp. Bot. 2001, 52, 1981–1989. [Google Scholar] [CrossRef]
- Ning, X.; Lin, M.; Huang, G.; Mao, J.; Gao, Z.; Wang, X. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front. Plant Sci. 2023, 14, 1190768. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, C.; Wang, R.; Shen, R.; Lan, P. Physiological and proteomic dissection of the rice roots in response to iron deficiency and excess. J. Proteom. 2022, 258, 104567. [Google Scholar] [CrossRef]
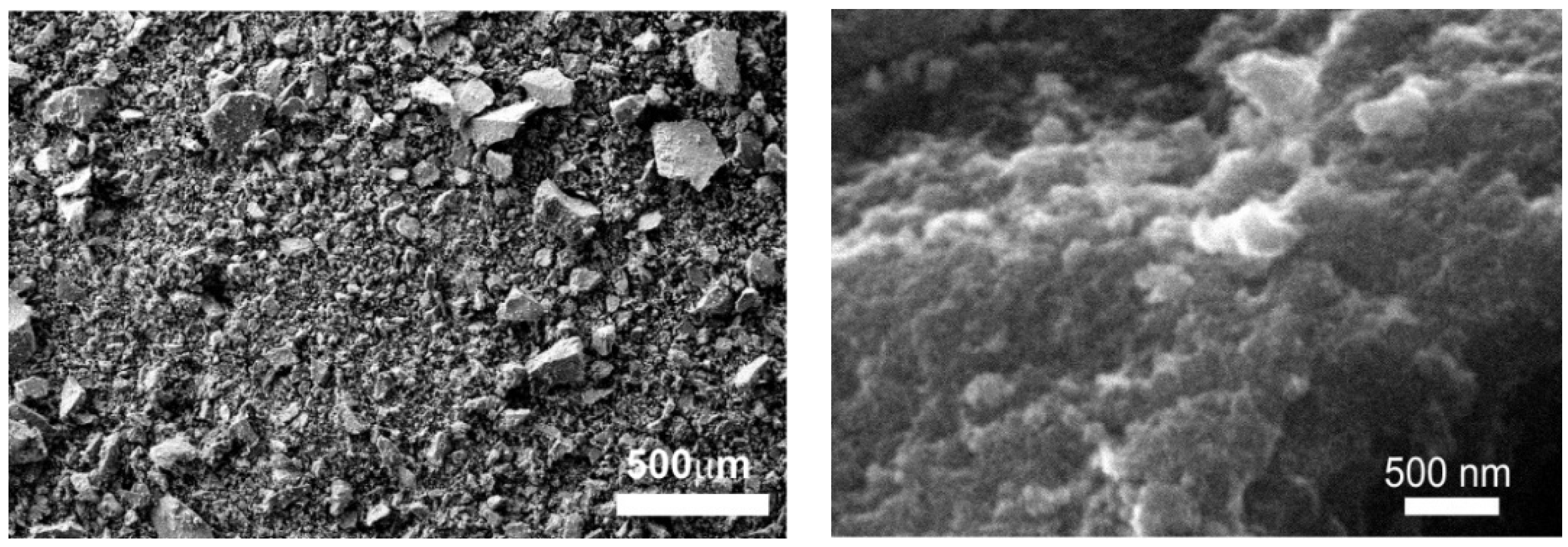
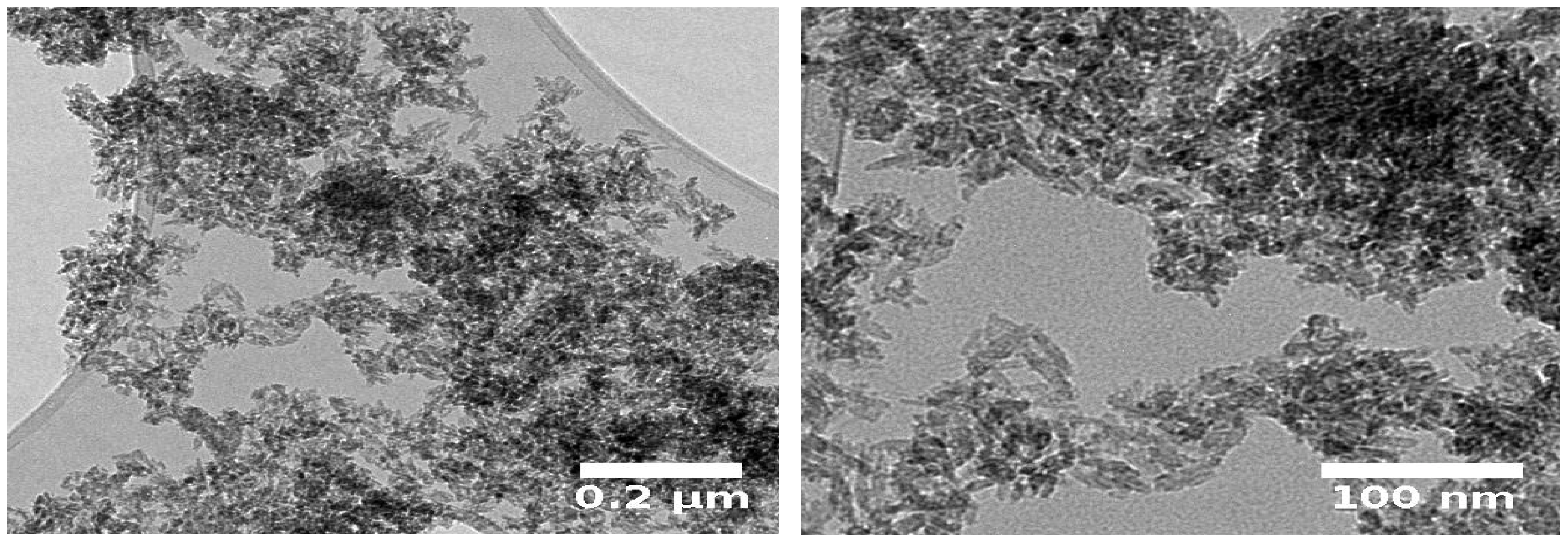
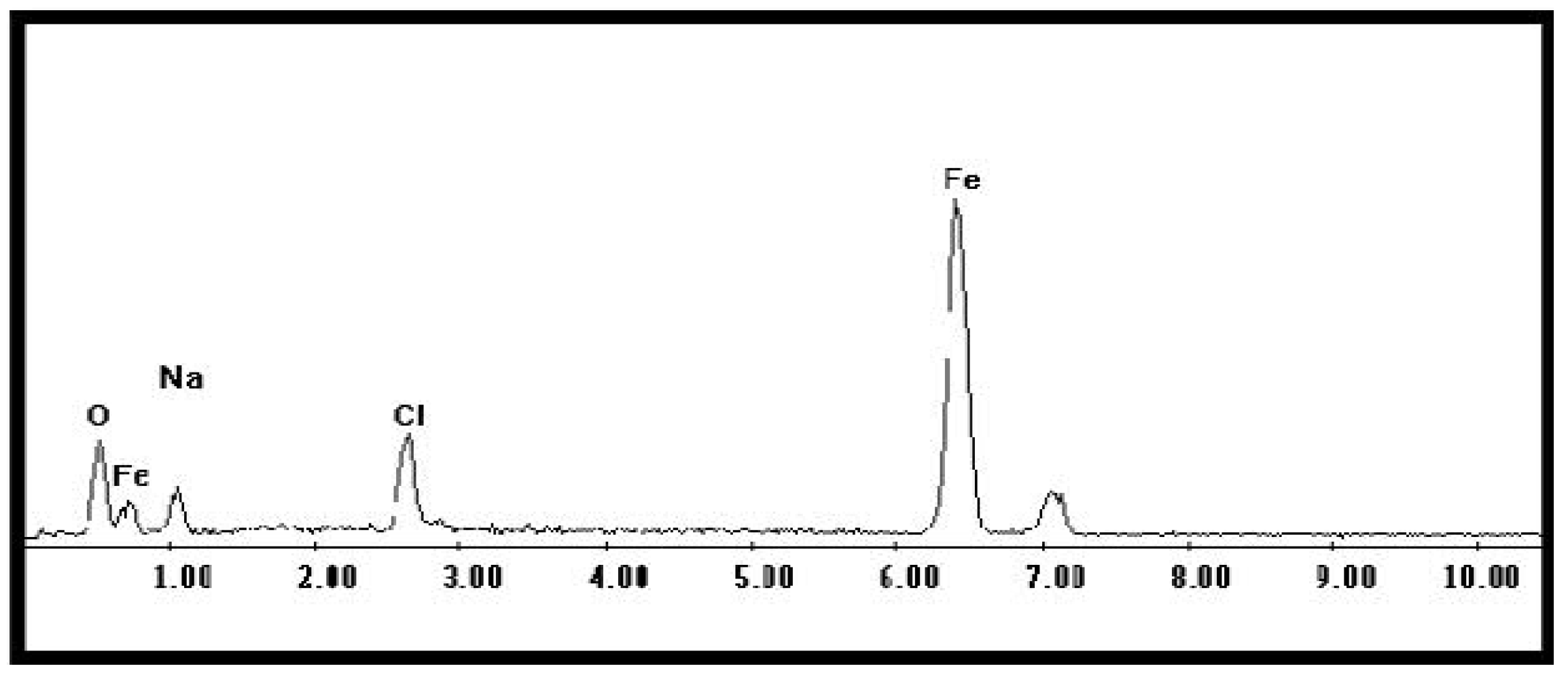

| Concentration (ppm) | Ferric Sulfate Fe2(SO4)3 | Iron Chelate (Fe-EDDHA) | IONPs (Fe2O3) |
|---|---|---|---|
| 0 | Control | Control | Control |
| 25 | FS25 | IC25 | IONPs25 |
| 50 | FS50 | IC50 | IONPs50 |
| 100 | FS100 | IC100 | IONPs100 |
| Treatment | Chlorophyll a μg cm−2 FW | Chlorophyll b μg cm−2 FW | Total Chlorophyll μg cm−2 FW | Carotenoids μg cm−2 FW |
|---|---|---|---|---|
| Control | 5.9938 ± 0.2335 ab | 2.5471 ± 0.1209 ab | 8.5409 ± 0.3545 ab | 1.0770± 0.0601 ab |
| IC25 | 5.2984 ± 0.2234 abc | 2.1760 ± 0.1242 abc | 7.4744 ± 0.3478 abc | 1.0249 ± 0.1242 abc |
| IC50 | 5.3067 ± 0.2150 abc | 2.2238 ± 0.0782 abc | 7.5306 ± 0.2932 abc | 0.9292 ± 0.0221 abc |
| IC100 | 6.4367 ± 1.0687 a | 2.9008 ± 0.8081 a | 9.3376 ± 1.8768 a | 1.0467 ± 0.8099 a |
| FS25 | 5.6351 ± 0.5159 abc | 2.3539 ± 0.2671 abc | 7.9890 ± 0.7830 abc | 1.1358 ± 0.1157 abc |
| FS50 | 5.3402 ± 1.1897 abc | 2.2876 ± 0.4731 abc | 7.6278 ± 1.6628 abc | 1.0773 ± 0.0001 abc |
| FS100 | 5.2843 ± 0.0244 abc | 2.2399 ± 0.1747 abc | 7.5242 ± 0.1991 abc | 0.9184 ± 0.0226 abc |
| IONPs25 | 6.5089 ± 0.4016 a | 2.8944 ± 0.2528 a | 9.4034 ± 0.6545 a | 1.2623 ± 0.2170 a |
| IONPs50 | 5.1449 ± 0.3743 bc | 2.0594 ± 0.2083 bc | 7.2044 ± 0.5826 bc | 1.0139 ± 0.0052 bc |
| IONPs100 | 3.7069 ± 0.0001 c | 1.4671 ± 0.1000 c | 5.1740 ± 0.0023 c | 0.7027 ± 0.0001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Lagos, C.L.; Navarro-León, E.; Ochoa-Chaparro, E.H.; Chávez-Mendoza, C.; Muñoz-Márquez, E.; Guevara-Aguilar, A.; Terrazas-Gómez, M.I.; Sánchez, E. Comparative Effects of Iron Nanoparticles, Chelates, and Iron Sulfate on Biomass, Yield, and Nitrogen Assimilation in Spinach. Nitrogen 2025, 6, 81. https://doi.org/10.3390/nitrogen6030081
Franco-Lagos CL, Navarro-León E, Ochoa-Chaparro EH, Chávez-Mendoza C, Muñoz-Márquez E, Guevara-Aguilar A, Terrazas-Gómez MI, Sánchez E. Comparative Effects of Iron Nanoparticles, Chelates, and Iron Sulfate on Biomass, Yield, and Nitrogen Assimilation in Spinach. Nitrogen. 2025; 6(3):81. https://doi.org/10.3390/nitrogen6030081
Chicago/Turabian StyleFranco-Lagos, Cristina L., Eloy Navarro-León, Erick H. Ochoa-Chaparro, Celia Chávez-Mendoza, Ezequiel Muñoz-Márquez, Alexandro Guevara-Aguilar, Marina I. Terrazas-Gómez, and Esteban Sánchez. 2025. "Comparative Effects of Iron Nanoparticles, Chelates, and Iron Sulfate on Biomass, Yield, and Nitrogen Assimilation in Spinach" Nitrogen 6, no. 3: 81. https://doi.org/10.3390/nitrogen6030081
APA StyleFranco-Lagos, C. L., Navarro-León, E., Ochoa-Chaparro, E. H., Chávez-Mendoza, C., Muñoz-Márquez, E., Guevara-Aguilar, A., Terrazas-Gómez, M. I., & Sánchez, E. (2025). Comparative Effects of Iron Nanoparticles, Chelates, and Iron Sulfate on Biomass, Yield, and Nitrogen Assimilation in Spinach. Nitrogen, 6(3), 81. https://doi.org/10.3390/nitrogen6030081








