Nutrient Recovery Strategies and Agronomic Performance in Circular Farming: A Comprehensive Review
Abstract
1. Introduction
| Metric | Value | Source |
|---|---|---|
| World population (2023) | 8.0 billion | [1] |
| Projected world population (2050) | 9.7 billion | [1] |
| Annual synthetic N fertilizer applied (global) | ~110 million t N | [8] |
| Phosphorus reserve lifetime | ~70 years | [9] |
| Potassium reserve lifetime | ~300 years | [10] |
| Regional share of N fertilizer use | Asia: 56%; Americas: 22%; Europe: 15%; Africa and Oceania: 7% | [8] |
| Annual manure generated in EU | 1.2–1.8 billion t | [11] |
| Annual crop residues in EU | 430 million t | [12] |
| Annual municipal wastewater generated | ~380 km3 | [13] |
| Annual sewage sludge production (dry solids) | ~45 million t | [14] |
2. Methodology
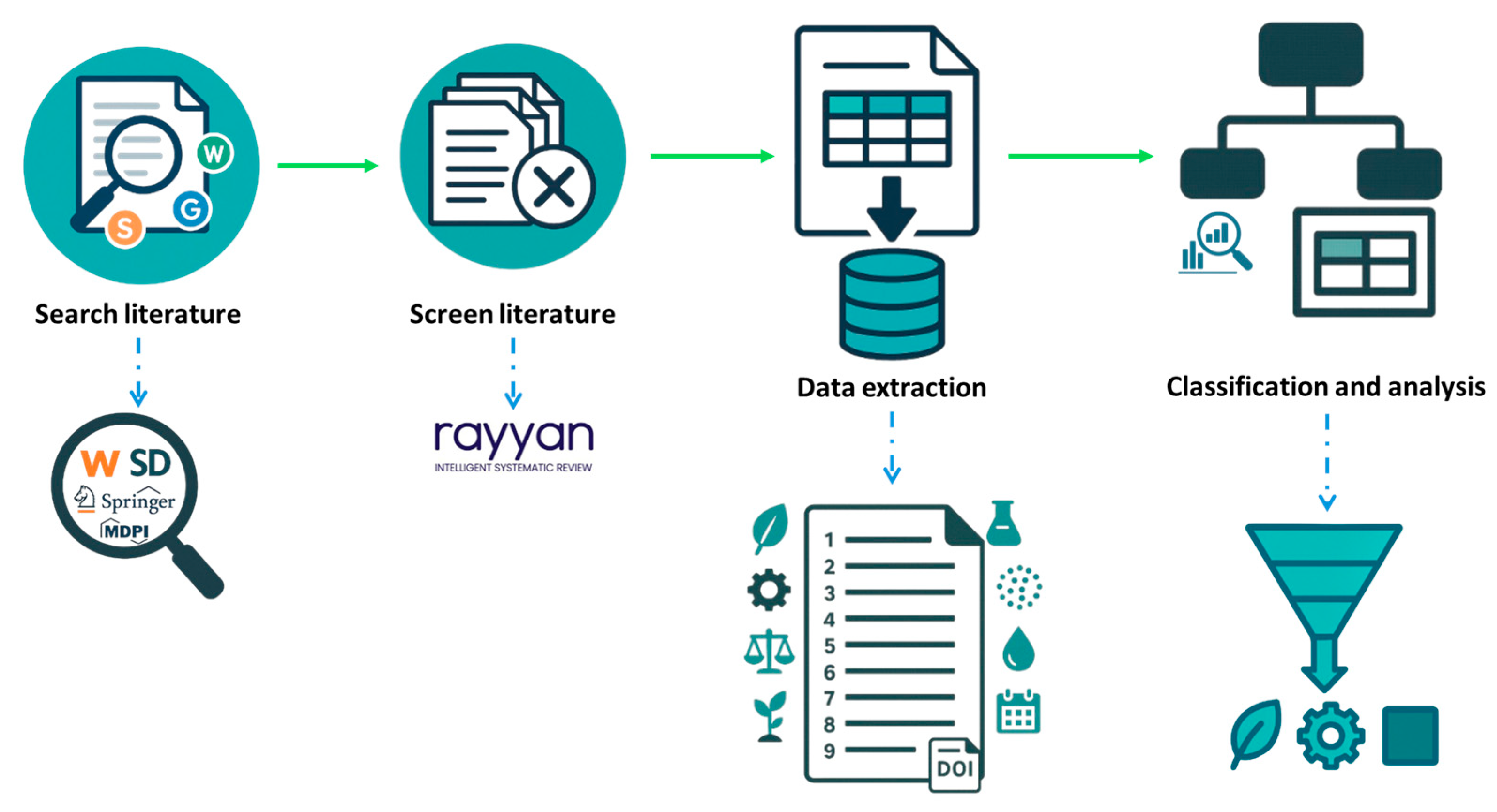
2.1. Data and Literature Sources
2.2. Study Selection
- i.
- The study had to refer to at least one product derived from waste.
- ii.
- The study must report the yield/biomass of the tested product.
- iii.
- The publication year of the study should be between 2010 and 2024.
- iv.
- If the language of study is English, Dutch, French, German, or Spanish, it should be included.
- i.
- Studies only considering technological aspects for producing bio-based product were not considered.
- ii.
- Studies only considering only primary treatments of waste streams (separation, digestate) were not considered.
- iii.
- Studies reporting heavy metal uptake using waste products were not considered.
- iv.
- Studies involving microorganisms for improving of fertilizer performance were not considered.
- v.
- Year of publication: any study carried out before 2014 was not included.
- vi.
- Language of study: papers available only in Japanese and Chinese languages were excluded.
- vii.
- Type of publication: review studies and book chapters were not considered.
- viii.
- Studies with an irrelevant abstract or with no full text available through any source were discarded.
2.3. Data Extraction
2.4. Overview of Data Collection
- In the initial phase, data (n = 86) were sorted according to the nutrient recovered from a particular study.
- Phase 2 of the literature survey involved categorizing the types of technology used for nutrient recovery reported in the studies.
- In the 3rd phase of scrutinization, the focus shifted to the products recovered from each technology and their agronomic performance in field and pot trials (Table 2).
2.5. Review Limitations
2.6. Data Analysis
3. Results and Discussion
3.1. Ammonium-Based Salts for N Recovery
3.1.1. Ammonium Sulfate (AS)
3.1.2. Ammonium Nitrate (AN)
3.1.3. Ammonia Water (AW)
3.2. Potassium-Based Extracts
3.2.1. Potassium Concentrate (KC)
3.2.2. Waste Mica (WM)
3.3. Organo-Mineral Phosphate Fertilizers (OMFs)
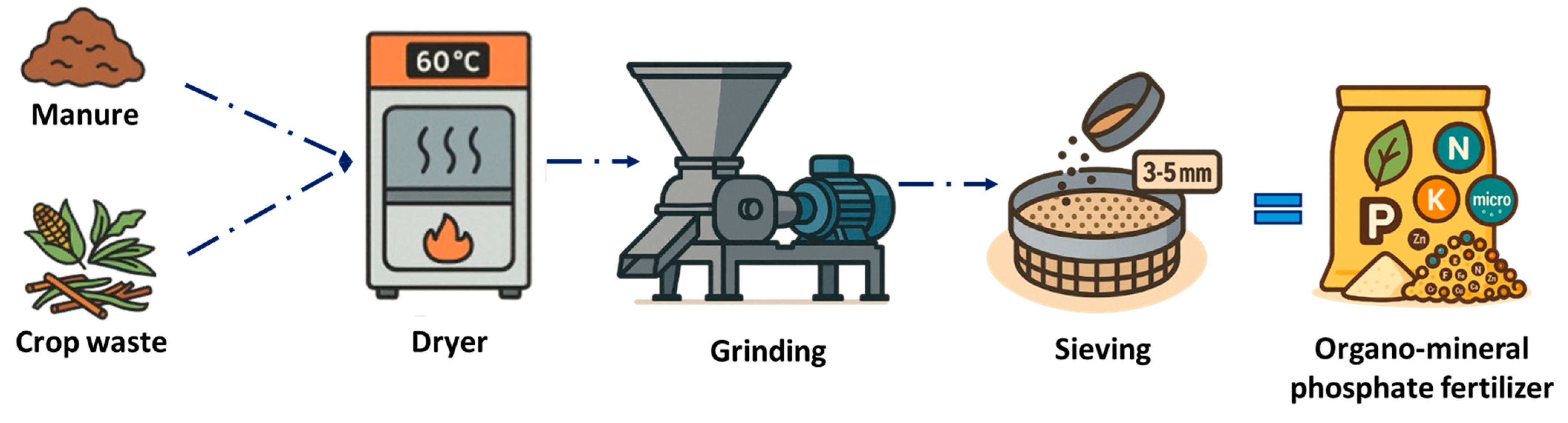
3.3.1. Manure-Derived OMF
3.3.2. Crop Waste-Derived OMF
3.4. Chars
3.4.1. Phosphorous-Rich Ash
3.4.2. Hydrochar
3.4.3. Biochar
3.5. Struvite
3.5.1. Struvite Derived from Wastewater Systems
3.5.2. Struvite Derived from Manure
3.6. Biostimulants
3.7. Sustainability and Market Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AN | Ammonium Nitrate |
| AS | Ammonium Sulfate |
| AW | Ammonia Water (Ammonium hydroxide, NH4OH) |
| BBF | Bio-Based Fertilizer |
| CAN | Calcium Ammonium Nitrate |
| CMC | Component Material Category (EU Fertilizer Regulation) |
| EC | European Commission |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| FPR | Fertilizer Products Regulation (EU 2019/1009) |
| IWA | International Water Association |
| JRC | Joint Research Centre (European Commission) |
| NDVI | Normalized Difference Vegetation Index |
| NNI | Nitrogen Nutrition Index |
| NPK | Nitrogen, Phosphorus, Potassium |
| OMF | Organo-Mineral Fertilizer |
| PFC | Product Function Category (EU Fertilizer Regulation) |
| RENURE | REcovered Nitrogen from manURE |
| UAV | Unmanned Aerial Vehicle |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| WWAP | World Water Assessment Programme (UNESCO) |
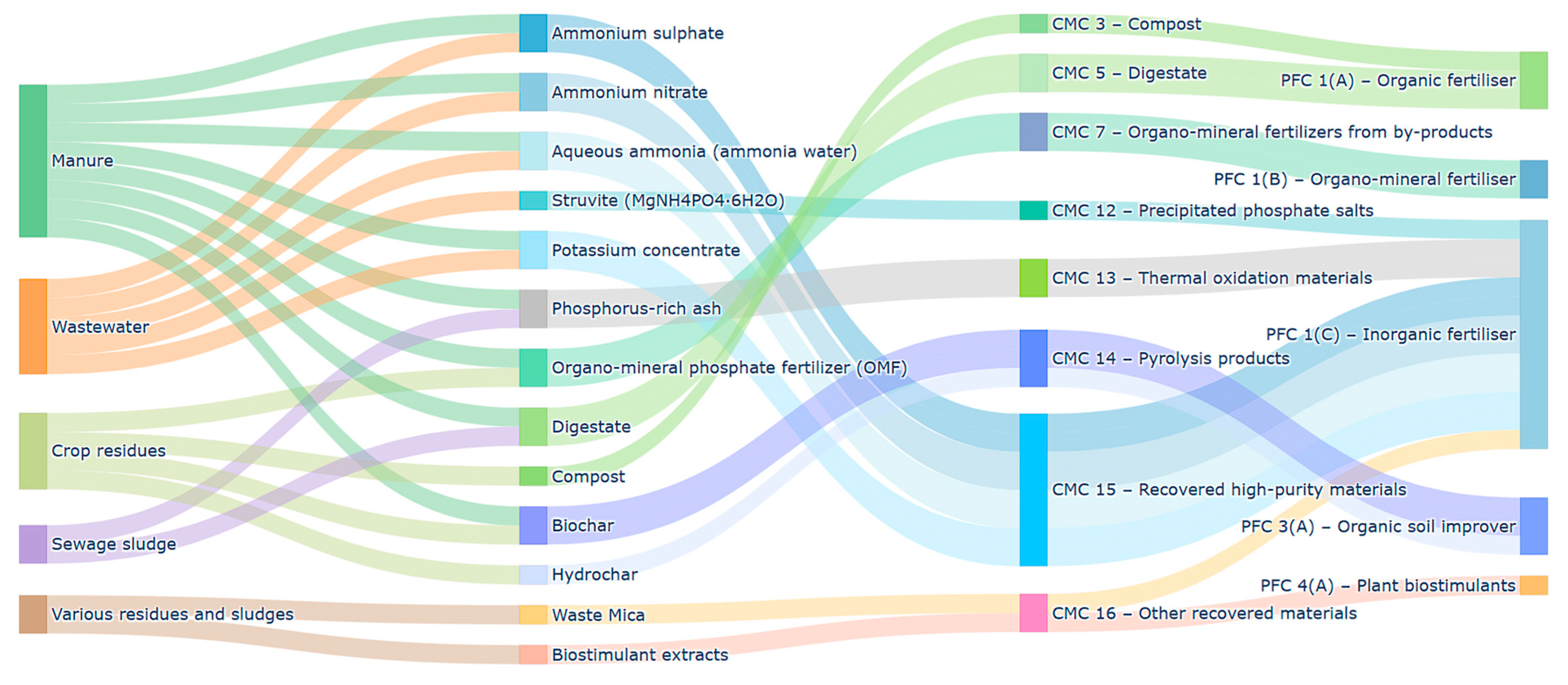
Appendix A. Detailed Classification of EU FPR
| Product | Source Stream(s) | CMC (Component Material Category) | PFC (Product Function Category) |
|---|---|---|---|
| Ammonium sulfate | Manure, wastewater | CMC 15 (Recovered high-purity materials) | PFC 1(C)(I)(a)(i)—Solid inorganic macronutrient fertilizer |
| Ammonium nitrate | Manure, wastewater | CMC 15 (Recovered high-purity materials) | PFC 1(C)(I)(a)(i)—Solid inorganic macronutrient fertilizer |
| Aqueous ammonia (“ammonia water”) | Manure, wastewater | CMC 15 (Recovered high-purity materials) | PFC 1(C)(I)(a)(ii)—Liquid inorganic macronutrient fertilizer |
| Struvite (MgNH4PO4·6H2O) | Municipal wastewater | CMC 12 (Precipitated phosphate salts) | PFC 1(C)(I)(a)(i)—Solid inorganic macronutrient fertilizer |
| Organo-mineral phosphate fertilizer (OMF) | Crop residues, manure | CMC 7 (Organo-mineral fertilizers from by-products) | PFC 1(C)(I)(b)—Solid mineral/organo-mineral macronutrient fertilizer |
| Phosphorus-rich ash | Sewage sludge ash, manure ash | CMC 13 (Thermal oxidation materials) | PFC 1(C)(I)(b)—Solid synthetic fertilizer |
| Potassium concentrate | Manure, wastewater | CMC 15 (Recovered high-purity materials) | PFC 1(C)(I)(a)(ii)—Liquid inorganic macronutrient fertilizer |
| Waste Mica | Mica mining waste | CMC 16 (Other recovered minerals) | PFC 1(C)(I)(b)—Solid synthetic fertilizer |
| Biochar | Crop residues, manure | CMC 14 (Pyrolysis products) | PFC 3(A)—Organic soil improver |
| Hydrochar | Crop residues | CMC 14 (Pyrolysis products) | PFC 3(A)—Organic soil improver |
| Compost | Crop residues, food waste | CMC 3 (Compost) | PFC 1(A)—Organic fertilizer |
| Digestate | Manure, sewage sludge | CMC 5 (Digestate) | PFC 1(A)—Organic fertilizer |
| Biostimulant extracts | Various residues and sludges | CMC 16 (Other recovered organics) | PFC 4(A)—Plant biostimulant |
References
- United Nations Department of Economic and Social Affairs. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. 21 June 2017. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 6 August 2025).
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Alternative Pathways to 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Loi, A.; Gentile, M.; Bradley, D.; Christodoulou, M.; Bracken, J.; Knuuttila, M.; Niemi, J.; Wejberg, H.; Nègre, F.; Bergman, J.; et al. Research for AGRI Committees: The Dependency of the EU’s Food System on Inputs and Their Sources (PE 747.272); European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2024; Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2024/747272/IPOL_STU%282024%29747272_EN.pdf (accessed on 6 August 2025).
- United Nations Environment Programme. Beat Nitrogen Pollution; United Nations Environment Programme: Nairobi, Kenya, 2023; Available online: https://www.unep.org/interactives/beat-nitrogen-pollution/ (accessed on 6 August 2025).
- Juncal, M.J.L.; Masino, P. Towards Nutrient Neutrality: A Review of Agricultural Runoff Mitigation Strategies and the Development of a Decision-Making Framework. Sci. Total Environ. 2023, 874, 162408. [Google Scholar] [CrossRef]
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S.; Comero, S.; Jones, A.; Gawlik, B.; Saveyn, H.G.M. Technical Proposals for the Safe Use of Processed Manure Above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC); Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. OJ L 170, 25 June 2019. pp. 1–114. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj/eng (accessed on 6 August 2025).
- Food and Agriculture Organization of the United Nations (FAO). Inorganic Fertilizers 2002–2022; FAO STAT Analytical Briefs; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Glob. Environ. Change 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Blanco, M.A. Supply of and Access to Key Nutrients NPK for Fertilizers for the Sustainability of EU Agricultural Production; JRC Scientific and Technical Reports; European Commission: Luxembourg, 2011. [Google Scholar]
- Vingerhoets, R. Circular Nutrient Management in Livestock-Dense Regions: Insights from Substance Flows and Cost-Benefit Analysis. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2024. Available online: http://hdl.handle.net/1854/LU-01JA8WNEF7BQ8E929HNF2DB125 (accessed on 6 August 2025).
- European Commission, Joint Research Centre. Study on the Availability of Agricultural Residues in the European Union; JRC Technical Report; European Commission: Luxembourg, 2024. [Google Scholar]
- UNESCO World Water Assessment Programme (WWAP). The United Nations World Water Development Report 2017: Wastewater, the Untapped Resource; UNESCO: Paris, France, 2017. [Google Scholar]
- International Water Association (IWA). Global Sewage Sludge Production, Fate and Treatment; IWA Publishing: London, UK, 2020. [Google Scholar]
- Sigurnjak, I.; Schlegel, J.; Roudier, S.; Hofmann, H.; Meers, E. Production and performance of bio-based mineral fertilizers from agricultural waste using ammonia stripping–scrubbing technology. Waste Manag. 2019, 95, 242–253. [Google Scholar] [CrossRef]
- Vingerhoets, R.; Brienza, C.; Sigurnjak, I.; Vlaeminck, S.E.; Spiller, M.; Meers, E. Ammonia stripping and scrubbing followed by nitrification-denitrification saves costs for manure treatment: A calibrated model approach. Chem. Eng. J. 2023, 477, 146984. [Google Scholar] [CrossRef]
- Saju, A.; Vlaeminck, S.E.; Ronsse, F.; Meers, E.; Vervaeren, H. Digestate-Derived Ammonium Fertilizers and Their Blends as Substitutes to Synthetic Nitrogen Fertilizers. Appl. Sci. 2022, 12, 3787. [Google Scholar] [CrossRef]
- Rietra, R.; Van Dijk, K.; Schoumans, O. Environmental effects of using ammonium sulfate from animal manure scrubbing technology as fertilizer. Appl. Sci. 2024, 14, 4998. [Google Scholar] [CrossRef]
- Horta, C.; Riaño, B.; Anjos, O.; García-González, M.C. Fertiliser effect of ammonia recovered from anaerobically digested orange peel using gas-permeable membranes. Sustainability 2022, 14, 7832. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lund, R.J.; Ter Heijne, A.; Sleutels, T.; Buisman, C.J.N.; Kuntke, P. Application of ammonium fertilizers recovered by an Electrochemical System. Resour. Conserv. Recycl. 2022, 181, 106225. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Michels, E.; Crappé, S.; Buysens, S.; Tack, F.M.G.; Meers, E. Utilization of derivatives from nutrient recovery processes as alternatives for fossil-based mineral fertilizers in commercial greenhouse production of Lactuca sativa L. Sci. Hortic. 2016, 198, 267–276. [Google Scholar] [CrossRef]
- Shrivastava, V. Evaluation of Performance and Efficiency of Bio-Based and Tailor-Made Fertilisers in Selected Nutrient Vulnerable Regions of the EU. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2024. Available online: http://hdl.handle.net/1854/LU-01JBVZC83E37YNPDHM5KJX1RF0 (accessed on 6 August 2025).
- Hendriks, C.M.J.; Shrivastava, V.; Sigurnjak, I.; Lesschen, J.P.; Meers, E.; Noort, R.V.; Yang, Z.; Rietra, R.P.J.J. Replacing mineral fertilisers for bio-based fertilisers in potato growing on sandy soil: A case study. Appl. Sci. 2021, 12, 341. [Google Scholar] [CrossRef]
- Jin, K.; Pezzuolo, A.; Gouda, S.G.; Jia, S.; Eraky, M.; Ran, Y.; Chen, M.; Ai, P. Valorization of bio-fertilizer from anaerobic digestate through ammonia stripping process: A practical and sustainable approach towards circular economy. Environ. Technol. Innov. 2022, 27, 102414. [Google Scholar] [CrossRef]
- Saju, A.; Van De Sande, T.; Ryan, D.; Karpinska, A.; Sigurnjak, I.; Dowling, D.N.; Germaine, K.; Kakouli-Duarte, T.; Meers, E. Exploring the short-term in-field performance of Recovered Nitrogen from Manure (Renure) materials to substitute synthetic nitrogen fertilisers. Clean. Circ. Bioecon. 2023, 5, 100043. [Google Scholar] [CrossRef]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Herrera, A.; Tambone, F.; Geromel, G.; Meers, E.; Schoumans, O.; Giordano, A.; Adani, F. Using highly stabilized digestate and digestate-derived ammonium sulphate to replace synthetic fertilizers: The effects on soil, environment, and crop production. Sci. Total Environ. 2022, 815, 152919. [Google Scholar] [CrossRef] [PubMed]
- Vaneeckhaute, C.; Ghekiere, G.; Michels, E.; Vanrolleghem, P.A.; Tack, F.; Meers, E. The use of digestates and recovered ammonium sulfate from NH3-scrubbing as sustainable substitutes for chemical fertilizers: A field-scale assessment. Global Challenges: Sustainable Wastewater Treatment and Resource Recovery, IWA Specialist Conference, Papers. In Proceedings of the IWA Specialist Conference on Global Challenges: Sustainable Wastewater Treatment and Resource Recovery, Kathmandu, Nepal, 26–30 October 2014; Available online: http://hdl.handle.net/1854/LU-5715322 (accessed on 6 August 2025).
- Powlson, D.S.; Dawson, C.J. Use of ammonium sulphate as a sulphur fertilizer: Implications for ammonia volatilization. Soil Use Manag. 2022, 38, 622–634. [Google Scholar] [CrossRef]
- Martin, J.W.; Moore, P.A.; Li, H.; Ashworth, A.J.; Miles, D.M. Effects of land-applied ammonia scrubber solutions on yield, nitrogen uptake, soil test phosphorus, and phosphorus runoff. J. Environ. Qual. 2018, 47, 263–269. [Google Scholar] [CrossRef]
- Müller, B.; Hartung, J.; von Cossel, M.; Lewandowski, I.; Müller, T.; Bauerle, A. On-farm use of recycled liquid ammonium sulphate in Southwest Germany using a participatory approach. Nutr. Cycl. Agroecosyst. 2024, 129, 459–474. [Google Scholar] [CrossRef]
- Ashitha, A.; Rakhimol, K.R. Fate of the conventional fertilizers in environment. In Controlled Release Fertilizers for Sustainable Agriculture; Academic Press: London, UK, 2021; pp. 25–39. [Google Scholar]
- Libutti, A.; Monteleone, M. Soil vs. groundwater: The quality dilemma. Managing nitrogen leaching and salinity control under irrigated agriculture in Mediterranean conditions. Agric. Water Manag. 2017, 186, 40–50. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Grunert, O.; Meers, E.; Jablonowski, N.D. Evaluating the fertilising potential of blended recovered nutrients in horticultural growing medium on Viola x wittrockiana L. Agronomy 2022, 12, 182. [Google Scholar] [CrossRef]
- Stofberg, S.F.; Klimkowska, A.; Paulissen, M.P.; Witte, J.P.M.; van der Zee, S.E. Effects of salinity on growth of plant species from terrestrializing fens. Aquat. Bot. 2015, 121, 83–90. [Google Scholar] [CrossRef]
- Carreras-Sempere, M.; Caceres, R.; Viñas, M.; Biel, C. Use of recovered struvite and ammonium nitrate in fertigation in tomato (Lycopersicum esculentum) production for boosting circular and sustainable horticulture. Agriculture 2021, 11, 1063. [Google Scholar] [CrossRef]
- Carreras-Sempere, M.; Biel, C.; Viñas, M.; Guivernau, M.; Caceres, R. The use of recovered struvite and ammonium nitrate in fertigation in a horticultural rotation: Agronomic and microbiological assessment. Environ. Technol. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Harty, M.A.; Forrestal, P.J.; Watson, C.J.; McGeough, K.L.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.J.; Richards, K.G.; Lanigan, G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016, 563, 576–586. [Google Scholar] [CrossRef]
- Fabbri, C.; Delgado, A.; Guerrini, L.; Napoli, M. Precision nitrogen fertilization strategies for durum wheat: A sustainability evaluation of NNI and NDVI map-based approaches. Eur. J. Agron. 2025, 164, 127502. [Google Scholar] [CrossRef]
- Tripolskaja, L.; Verbylienė, I. The effect of different forms of nitrogen fertilizers on nitrogen leaching. Zemdirbyste-Agriculture 2014, 101, 243–248. [Google Scholar] [CrossRef][Green Version]
- Nikolajsen, M.T.; Pacholski, A.S.; Sommer, S.G. Urea ammonium nitrate solution treated with inhibitor technology: Effects on ammonia emission reduction, wheat yield, and inorganic N in soil. Agronomy 2020, 10, 161. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, B. Purification technologies for NOx removal from flue gas: A review. Separations 2022, 9, 307. [Google Scholar] [CrossRef]
- Shrivastava, V.; Sigurnjak, I.; Edayilam, N.; Meers, E. Ammonia water as a biobased fertiliser: Evaluating agronomic and environmental performance for Lactuca sativa compared to synthetic fertilisers. Biocatal. Agric. Biotechnol. 2023, 54, 102907. [Google Scholar] [CrossRef]
- Luo, H.; Dewitte, K.; Landschoot, S.; Sigurnjak, I.; Robles-Aguilar, A.A.; Michels, E.; De Neve, S.; Haesaert, G.; Meers, E. Benefits of biobased fertilizers as substitutes for synthetic nitrogen fertilizers: Field assessment combining minirhizotron and UAV-based spectrum sensing technologies. Front. Environ. Sci. 2022, 10, 988932. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere effect on nutrient availability in soil and its uptake by plants: A review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Soil Fertility and Fertilisers|Growing Maize|Corson Maize. Available online: https://www.corsonmaize.co.nz/growing-maize/soil-fertility-and-fertiliser#:~:text=Maize%20requires%20a%20soil%20pH,K%20and%20molybdenum%20availability%20falls (accessed on 6 August 2025).
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Connor, R.; Feldgarden, M.; Fine, A.M.; Funk, K.; Hoffman, J.; et al. Database resources of the national center for biotechnology information in 2025. Nucleic Acids Res. 2025, 53, D20–D29. [Google Scholar] [CrossRef]
- Luo, H.; Zilio, M.; Sigurnjak, I.; Robles-Aguilar, A.A.; Michels, E.; Adani, F.; De Neve, S.; Meers, E. Dynamics of soil nitrogen and N-cycling-related genes following the application of biobased fertilizers. Appl. Soil Ecol. 2023, 191, 105033. [Google Scholar] [CrossRef]
- Basak, B.B. Waste mica as alternative source of plant-available potassium: Evaluation of agronomic potential through chemical and biological methods. Nat. Resour. Res. 2019, 28, 953–965. [Google Scholar] [CrossRef]
- Biswas, S.S.; Biswas, D.R.; Sarkar, A.; Ghosh, A. Oxalic-acid-treated waste mica, a potent natural supplement to k fertilizers for growing wheat and rice in inceptisol. J. Soil Sci. Plant Nutr. 2023, 23, 581–593. [Google Scholar] [CrossRef]
- Pramanik, P.; Kalita, C.; Borah, K.; Kalita, P. Combined application of mica waste and Bacillus pseudomycoides as a potassium solubilizing bio-fertilizer reduced the dose of potassium fertilizer in tea-growing soil. Agroecol. Sustain. Food Syst. 2021, 45, 732–744. [Google Scholar] [CrossRef]
- Ding, Z.J.; Shi, Y.Z.; Li, G.X.; Harberd, N.P.; Zheng, S.J. Tease out the future: How tea research might enable crop breeding for acid soil tolerance. Plant Commun. 2021, 2, 100182. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Boukhari, M.E.M.E.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of waste into organo-mineral fertilizers: Current technological trends and prospects. Rev. Environ. Sci. Bio/Technol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- de Melo Benites, V.; Dal Molin, S.J.; Menezes, J.F.S.; Guimarães, G.S.; de Almeida Machado, P.L.O. Organomineral fertilizer is an agronomic efficient alternative for poultry litter phosphorus recycling in an acidic ferralsol. Front. Agron. 2022, 4, 785753. [Google Scholar] [CrossRef]
- Sá, J.M.e.; Jantalia, C.P.; Teixeira, P.C.; Polidoro, J.C.; Benites, V.d.M.; Araújo, A.P. Agronomic and P recovery efficiency of organomineral phosphate fertilizer from poultry litter in sandy and clayey soils. Pesqui. Agropecuária Bras. 2017, 52, 786–793. [Google Scholar] [CrossRef]
- Frazão, J.J.; Benites, V.D.M.; Ribeiro, J.V.S.; Pierobon, V.M.; Lavres, J. Agronomic effectiveness of a granular poultry litter-derived organomineral phosphate fertilizer in tropical soils: Soil phosphorus fractionation and plant responses. Geoderma 2019, 337, 582–593. [Google Scholar] [CrossRef]
- Sakurada, L.R.; Batista, M.A.; Inoue, T.T.; Muniz, A.S.; Pagliari, P.H. Organomineral phosphate fertilizers: Agronomic efficiency and residual effect on initial corn development. Agron. J. 2016, 108, 2050–2059. [Google Scholar] [CrossRef]
- Nascimento, C.O.; Mattos, B.B.; Dal Molin, S.J.; Fialho, R.L.; Cabral-Albuquerque, E.C.M.; Benites, V.M. Phosphorus diffusion and agronomic efficiency of chicken litter organomineral fertilizers improved with binder materials. Waste Biomass Valorization 2021, 12, 3765–3772. [Google Scholar] [CrossRef]
- Sitzmann, T.J.; Alpigiano, A.; Lerda, C.; Moretti, B.; Zavattaro, L.; Grignani, C. Response of tomato to innovative organo-mineral fertilizers. Front. Sustain. Food Syst. 2024, 8, 1385828. [Google Scholar] [CrossRef]
- Vieira, D.M.D.S.; Torres, J.L.R.; Camargo, R.D.; Silva, A.D.A.; Lana, R.M.Q.; Charlo, H.C.D.O.; Lemes, E.M.; Carvalho, É.R. Residual effects of phosphorus and micronutrients in vegetable growing areas under different organomineral fertilizer doses. Horticulturae 2023, 9, 761. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Erenoğlu, E.B.; Morsy Mohammed Morsy, M.E.; Dündar, Ş. The effect of organomineral fertilizer phosphorus on the availability of phosphorus in a calcareous soil. Appl. Ecol. Environ. Res. 2023, 21, 4545–4562. [Google Scholar] [CrossRef]
- Magela, M.L.M.; Luz, J.M.Q.; Lana, R.M.Q.; Oliveira, R.C.D.; Gontijo, L.N.; Finzi, R.R.; Maciel, G.M.; Siquieroli, A.C.S. Organomineral fertilizer in planting of potato cultivars ágata and atlantic. Agronomy 2025, 15, 1833. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Bai, J. Content, modes of occurrence, and significance of phosphorous in biomass and biomass ash. J. Energy Inst. 2023, 108, 101205. [Google Scholar] [CrossRef]
- Atienza–Martínez, M.; Gea, G.; Arauzo, J.; Kersten, S.R.; Kootstra, A.M.J. Phosphorus recovery from sewage sludge char ash. Biomass Bioenergy 2014, 65, 42–50. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Advances in design strategies for preparation of biochar based catalytic system for production of high value chemicals. Bioresour. Technol. 2020, 299, 122564. [Google Scholar] [CrossRef]
- Cabeza, R.A.; Steingrobe, B.; Claassen, N. Phosphorus fractionation in soils fertilized with recycled phosphorus products. J. Soil Sci. Plant Nutr. 2019, 19, 611–619. [Google Scholar] [CrossRef]
- Barrow, N.J.; Debnath, A. Effect of phosphate status on the sorption and desorption properties of some soils of northern India. Plant Soil 2014, 378, 383–395. [Google Scholar] [CrossRef]
- Bogdan, A.; Aguilar, A.A.R.; Nys, O.; Michels, E.; Meers, E. Phosphorus availability in recycled fertilizers: Comparison of 11 chemical extraction methods with plant uptake during a 7-month growth experiment. J. Soil Sci. Plant Nutr. 2023, 23, 693–705. [Google Scholar] [CrossRef]
- Dombinov, V.; Herzel, H.; Meiller, M.; Müller, F.; Willbold, S.; Zang, J.W.; da Fonseca-Zang, W.A.; Adam, C.; Klose, H.; Poorter, H.; et al. Sugarcane bagasse ash as fertilizer for soybeans: Effects of added residues on ash composition, mineralogy, phosphorus extractability and plant availability. Front. Plant Sci. 2022, 13, 1041924. [Google Scholar] [CrossRef] [PubMed]
- de Jager, M.; Giani, L. An investigation of the effects of hydrochar application rate on soil amelioration and plant growth in three diverse soils. Biochar 2021, 3, 349–365. [Google Scholar] [CrossRef]
- de Jager, M.; Röhrdanz, M.; Giani, L. The influence of hydrochar from biogas digestate on soil improvement and plant growth aspects. Biochar 2020, 2, 177–194. [Google Scholar] [CrossRef]
- Huezo, L.; Shah, A. Effect of hydrochar from anaerobically digested sewage sludge and manure as a soil amendment on soil properties and plant responses. BioEnergy Res. 2023, 16, 1195–1204. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Suo, F.; You, X.; Yuan, Y.; Cheng, Y.; Zhang, C.; Li, Y. Effect of biochar and hydrochar from cow manure and reed straw on lettuce growth in an acidified soil. Chemosphere 2022, 298, 134191. [Google Scholar] [CrossRef]
- Reibe, K.; Roß, C.-L.; Ellmer, F. Hydro-/Biochar application to sandy soils: Impact on yield components and nutrients of spring wheat in pots. Arch. Agron. Soil Sci. 2015, 61, 1055–1060. [Google Scholar] [CrossRef]
- Melo, T.M.; Bottlinger, M.; Schulz, E.; Leandro, W.M.; Botelho De Oliveira, S.; Menezes De Aguiar Filho, A.; El-Naggar, A.; Bolan, N.; Wang, H.; Ok, Y.S.; et al. Management of biosolids-derived hydrochar (Sewchar): Effect on plant germination, and farmers’ acceptance. J. Environ. Manag. 2019, 237, 200–214. [Google Scholar] [CrossRef]
- Melo, T.M.; Bottlinger, M.; Schulz, E.; Leandro, W.M.; Menezes De Aguiar Filho, A.; Wang, H.; Ok, Y.S.; Rinklebe, J. Plant and soil responses to hydrothermally converted sewage sludge (Sewchar). Chemosphere 2018, 206, 338–348. [Google Scholar] [CrossRef]
- Bever, C.G.; Coronella, C.J. Carbon Sequestration Potential of Manure-Derived Hydrochar Aided by Secondary Stabilization. ACS Sustain. Chem. Eng. 2024, 12, 5705–5715. [Google Scholar] [CrossRef]
- Karatas, O.; Khataee, A.; Kalderis, D. Recent progress on the phytotoxic effects of hydrochars and toxicity reduction approaches. Chemosphere 2022, 298, 134357. [Google Scholar] [CrossRef]
- Krounbi, L.; Enders, A.; Gaunt, J.; Ball, M.; Lehmann, J. Plant uptake of nitrogen adsorbed to biochars made from dairy manure. Sci. Rep. 2021, 11, 15001. [Google Scholar] [CrossRef]
- Nardis, B.O.; Santana Da Silva Carneiro, J.; Souza, I.M.G.D.; Barros, R.G.D.; Azevedo Melo, L.C. Phosphorus recovery using magnesium-enriched biochar and its potential use as fertilizer. Arch. Agron. Soil Sci. 2021, 67, 1017–1033. [Google Scholar] [CrossRef]
- Carneiro, J.S.D.S.; Ribeiro, I.C.A.; Nardis, B.O.; Barbosa, C.F.; Lustosa Filho, J.F.; Melo, L.C.A. Long-term effect of biochar-based fertilizers application in tropical soil: Agronomic efficiency and phosphorus availability. Sci. Total Environ. 2021, 760, 143955. [Google Scholar] [CrossRef]
- Ali, I.; Ullah, S.; He, L.; Zhao, Q.; Iqbal, A.; Wei, S.; Shah, T.; Ali, N.; Bo, Y.; Adnan, M.; et al. Combined application of biochar and nitrogen fertilizer improves rice yield, microbial activity and N-metabolism in a pot experiment. PeerJ 2020, 8, e10311. [Google Scholar] [CrossRef]
- Kulczycki, G.; Magnucka, E.G.; Oksińska, M.P.; Kucińska, J.; Kobyłecki, R.; Pawęska, K.; Zarzycki, R.; Kacprzak, A.; Pietr, S.J. The effect of various types of biochar mixed with mineral fertilization on the development and ionome of winter wheat (Triticum aestivum L.) seedlings and soil properties in a pot experiment. Agronomy 2020, 10, 1903. [Google Scholar] [CrossRef]
- He, L.; Zhao, J.; Yang, S.; Zhou, H.; Wang, S.; Zhao, X.; Xing, G. Successive biochar amendment improves soil productivity and aggregate microstructure of a red soil in a five-year wheat-millet rotation pot trial. Geoderma 2020, 376, 114570. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Malicek, O.; Baltazar, T.; Latal, O.; Brtnicky, M. Manure maturation with biochar: Effects on plant biomass, manure quality and soil microbiological characteristics. Agriculture 2022, 12, 314. [Google Scholar] [CrossRef]
- Manolikaki, I.I.; Mangolis, A.; Diamadopoulos, E. The impact of biochars prepared from agricultural residues on phosphorus release and availability in two fertile soils. J. Environ. Manag. 2016, 181, 536–543. [Google Scholar] [CrossRef]
- De La Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef]
- Smider, B.; Singh, B. Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Manolikaki, I.; Diamadopoulos, E. Agronomic potential of biochar prepared from brewery byproducts. J. Environ. Manag. 2020, 255, 109856. [Google Scholar] [CrossRef] [PubMed]
- Manolikaki, I.; Diamadopoulos, E. Positive effects of biochar and biochar-compost on maize growth and nutrient availability in two agricultural soils. Commun. Soil Sci. Plant Anal. 2019, 50, 512–526. [Google Scholar] [CrossRef]
- Banik, C.; Koziel, J.A.; Bonds, D.; Singh, A.K.; Licht, M.A. Comparing biochar-swine manure mixture to conventional manure impact on soil nutrient availability and plant uptake—A greenhouse study. Land 2021, 10, 372. [Google Scholar] [CrossRef]
- Piri, M.; Sepehr, E. Struvite/biochar composites as recovered phosphorus fertilizers from domestic sewage sludge increased biomass and nutrient uptake of maize. J. Plant Nutr. 2024, 47, 433–447. [Google Scholar] [CrossRef]
- Fang, L.; Li, L.; Wang, Q.; Li, J.; Poon, C.S. Agronomic effectiveness of recovered phosphate fertilizer produced from incinerated sewage sludge ash. Waste Dispos. Sustain. Energy 2022, 4, 157–167. [Google Scholar] [CrossRef]
- Carella, F.; Seck, M.; Esposti, L.D.; Diadiou, H.; Maienza, A.; Baronti, S.; Vignaroli, P.; Vaccari, F.P.; Iafisco, M.; Adamiano, A. Thermal conversion of fish bones into fertilizers and biostimulants for plant growth—A low tech valorization process for the development of circular economy in least developed countries. J. Environ. Chem. Eng. 2021, 9, 104815. [Google Scholar] [CrossRef]
- Panten, K.; Leinweber, P. Agronomische Bewertung nach fünfjähriger Phosphordüngung mit Knochenkohle. J. Für Kult. 2020, 72, 561–576. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.J.; Novak, A.; Yang, Y.; Wang, J. Role of biochar in improving sandy soil water retention and resilience to drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Yang, M.; Wang, B.; Zhang, F. Biochar application reduces soil detachment capacity by overland flow under a continuous three-year field experiment on the Loess Plateau of China. Int. Soil Water Conserv. Res. 2025, 13, 687–701. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Polifka, S.; Fischer, D.; Glaser, B. Long-term biochar and soil organic carbon stability—Evidence from field experiments in Germany. Sci. Total Environ. 2024, 954, 176340. [Google Scholar] [CrossRef]
- Aka, R.J.N.; Hossain, M.; Yuan, Y.; Agyekum-Oduro, E.; Zhan, Y.; Zhu, J.; Wu, S. Nutrient recovery through struvite precipitation from anaerobically digested poultry wastewater in an air-lift electrolytic reactor: Process modeling and cost analysis. Chem. Eng. J. 2023, 465, 142825. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Xu, B.; Li, M.; Wang, J.; Shao, Y.; Chen, F.; Sun, M.; Liu, B. Effects of physicochemical parameters on struvite crystallization based on kinetics. Int. J. Environ. Res. Public Health 2022, 19, 7204. [Google Scholar] [CrossRef]
- Achilleos, P.; Roberts, K.R.; Williams, I.D. Struvite precipitation within wastewater treatment: A problem or a circular economy opportunity? Heliyon 2022, 8, e09862. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution rate and agronomic effectiveness of struvite fertilizers–effect of soil pH, granulation and base excess. Plant Soil 2017, 410, 139–152. [Google Scholar] [CrossRef]
- Thiessen Martens, J.R.; Entz, M.H.; Schneider, K.D.; Zvomuya, F.; Wilson, H.F. Response of organic grain and forage crops to struvite application in an alkaline soil. Agron. J. 2022, 114, 795–810. [Google Scholar] [CrossRef]
- Omidire, N.S.; Brye, K.R.; English, L.; Popp, J.; Kekedy-Nagy, L.; Greenlee, L.; Roberts, T.L.; Gbur, E.E. Wastewater-recovered struvite evaluation as a fertilizer-phosphorus source for corn in eastern Arkansas. Agron. J. 2022, 114, 2994–3012. [Google Scholar] [CrossRef]
- Mehta, C.M.; Hunter, M.N.; Leong, G.; Batstone, D.J. The value of wastewater derived struvite as a source of phosphorus fertilizer. CLEAN—Soil Air Water 2018, 46, 1700027. [Google Scholar] [CrossRef]
- Kokulan, V.; Schneider, K.; Macrae, M.L.; Wilson, H. Struvite application to field corn decreases the risk of environmental phosphorus loss while maintaining crop yield. Agric. Ecosyst. Environ. 2024, 366, 108936. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Dombinov, V.; Robles-Aguilar, A.A.; Jablonowski, N.D.; Ribeiro, C. Struvite-based composites for slow-release fertilization: A case study in sand. Sci. Rep. 2022, 12, 14176. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Shim, S.; Kim, S.; Ahmed, N.; Won, S.; Ra, C. Nutrient leaching loss of pre-treated struvite and its application in sudan grass cultivation as an eco-friendly and sustainable fertilizer source. Sustainability 2019, 11, 4204. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The effect of pH on morphological and physiological root traits of Lupinus angustifolius treated with struvite as a recycled phosphorus source. Plant Soil 2019, 434, 65–78. [Google Scholar] [CrossRef]
- Gong, W.; Li, Y.; Luo, L.; Luo, X.; Cheng, X.; Liang, H. Application of struvite-map crystallization reactor for treating cattle manure anaerobic digested slurry: Nitrogen and phosphorus recovery and crystal fertilizer efficiency in plant trials. Int. J. Environ. Res. Public Health 2018, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, diffusion and crop uptake of phosphorus in three different struvites. Sustainability 2018, 11, 134. [Google Scholar] [CrossRef]
- Liu, X.; Tao, Y.; Wen, G.; Kong, F.; Zhang, X.; Hu, Z. Influence of soil and irrigation water ph on the availability of phosphorus in struvite derived from urine through a greenhouse pot experiment. J. Agric. Food Chem. 2016, 64, 3324–3329. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Wąs, A.; Sosulski, T.; Van Pruissen, G.W.P.; Cornelissen, R.L. Struvite—An innovative fertilizer from anaerobic digestate produced in a bio-refinery. Energies 2019, 12, 296. [Google Scholar] [CrossRef]
- Szymańska, M.; Sosulski, T.; Szara, E.; Wąs, A.; Sulewski, P.; Van Pruissen, G.W.P.; Cornelissen, R.L. Ammonium sulphate from a bio-refinery system as a fertilizer—Agronomic and economic effectiveness on the farm scale. Energies 2019, 12, 4721. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant- and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Shrivastava, V.; Edayilam, N.; Singla Just, B.; Castaño-Sanchez, O.; Díaz-Guerra, L.; Meers, E. Evaluation of agronomic efficiency and stress resistance on Swiss chard via use of biostimulants. Sci. Hortic. 2024, 330, 113053. [Google Scholar] [CrossRef]
- Sleighter, R.L.; Hanson, T.; Holden, D.; Richards, K.M. Abiotic stress mitigation: A case study from 21 trials using a natural organic matter based biostimulant across multiple geographies. Agronomy 2023, 13, 728. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of biostimulants in tomato plants (Solanum lycopersicum) to enhance plant growth and salt stress tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides Derived from the Brown Algae Lessonia nigrescens Enhance Salt Stress Tolerance to Wheat Seedlings by Enhancing the Antioxidant System and Modulating Intracellular Ion Concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Fasani, E.; Franceschi, C.; Furini, A.; DalCorso, G. Effect of biostimulants combined with fertilization on yield and nutritional value of wheat crops. BMC Plant Biol. 2025, 25, 736. [Google Scholar] [CrossRef]
- Izquierdo, J.; Arriagada, O.; García-Pintos, G.; Ortiz, R.; García-Pintos, M.; García-Pintos, M. On-farm foliar application of a humic biostimulant increases the yield of rice. Agron. J. 2024, 116, 2551–2563. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Gurmani, Z.A.; Khan, S.; Khan, A.; Farid, A.; Khan, S.; Hameed, M.U. Optimization of biostimulants application for phenology and quality of oats. Braz. Arch. Biol. Technol. 2021, 64, e21200726. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. S. Afr. J. Bot. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Khouloud, M.; Farrie, Y.; Boulif, R.; Kadmiri, I.M.; Bamouh, A.; Douira, A. Improving growth, yield, and quality of tomato plants (Solanum lycopersicum L.) by the application of moroccan seaweed-based biostimulants under greenhouse conditions. Agronomy 2021, 11, 1373. [Google Scholar] [CrossRef]
- Rajendran, R.; Jagmohan, S.; Jayaraj, P.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Effects of Ascophyllum nodosum extract on sweet pepper plants as an organic biostimulant in grow box home garden conditions. J. Appl. Phycol. 2022, 34, 647–657. [Google Scholar] [CrossRef]
- Žižková, E.; Dobrev, P.I.; Muhovski, Y.; Hošek, P.; Hoyerová, K.; Haisel, D.; Procházková, D.; Lutts, S.; Motyka, V.; Hichri, I. Tomato (Solanum lycopersicum L.) SlIPT3 and SlIPT4 isopentenyltransferases mediate salt stress response in tomato. BMC Plant Biol. 2015, 15, 132–140. [Google Scholar] [CrossRef]
- Wadas, W.; Dziugieł, T. Changes in assimilation area and chlorophyll content of very early potato (Solanum tuberosum L.) cultivars as influenced by biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef]
- Kolachevskaya, O.O.; Lomin, S.N.; Arkhipov, D.V.; Romanov, G.A. Auxin in potato: Molecular aspects and emerging roles in tuber formation and stress resistance. Plant Cell Rep. 2019, 38, 681–698. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, X.; Bao, X.; Lam, K.L. Life cycle assessment of ammonium sulfate recovery from urban wastewater. Blue-Green Syst. 2024, 6, 90–99. [Google Scholar] [CrossRef]
- Martín-Hernández, E.; Montero-Rueda, C.; Ruiz-Mercado, G.J.; Vaneeckhaute, C.; Martín, M. Multi-scale techno-economic assessment of nitrogen recovery systems for livestock operations. Sustain. Prod. Consum. 2023, 41, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Latawiec, A.E.; Rodrigues, A.F.; Korys, K.A.; Mendes, M.; Rangel, M.; Castro, A.; Teixeira, W.; Valentim, J.F.; Araujo, E.; de Moraes, L.F.D.; et al. Biochar and Forage Peanut improve pastures: Evidence from a field experiment in Brazil. Agric. Ecosyst. Environ. 2023, 353, 108534. [Google Scholar] [CrossRef]
- Woolf, D.; Lehmann, J.; Ogle, S.; Kishimoto-Mo, A.W.; McConkey, B.; Baldock, J. Greenhouse gas inventory model for biochar additions to soil. Environ. Sci. Technol. 2021, 55, 14795–14805. [Google Scholar] [CrossRef]
- Anyebe, O.; Sadiq, F.K.; Manono, B.O.; Matsika, T.A. Biochar characteristics and application: Effects on soil ecosystem services and nutrient dynamics for enhanced crop yields. Nitrogen 2025, 6, 31. [Google Scholar] [CrossRef]
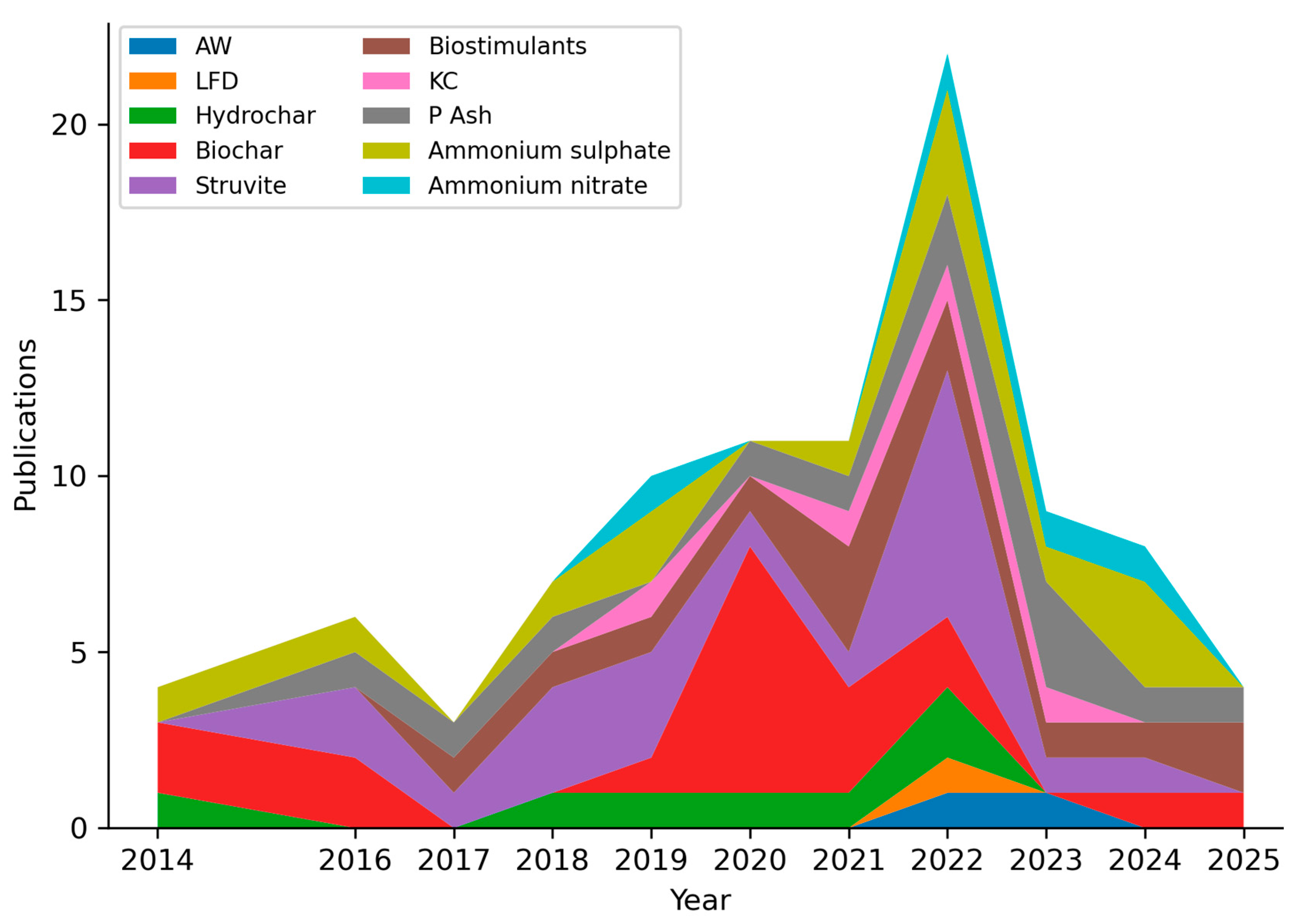


| Nutrient Recovered | Product Obtained | Number of Studies |
|---|---|---|
| Nitrogen (N) | Ammonium sulfate | 15 |
| Ammonium nitrate | 7 | |
| Ammonia water | 3 | |
| Potassium (K) | Potassium concentrate | 3 |
| Waste mica | 3 | |
| Organo-mineral phosphate fertilizer | 8 | |
| Phosphorous (P) | Phosphorous rich ash | 3 |
| Hydrochar | 7 | |
| Biochar | 19 | |
| Struvite | 18 | |
| Others | Biostimulants | 13 |
| Total | 86 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastava, V.; Laasri, I. Nutrient Recovery Strategies and Agronomic Performance in Circular Farming: A Comprehensive Review. Nitrogen 2025, 6, 80. https://doi.org/10.3390/nitrogen6030080
Shrivastava V, Laasri I. Nutrient Recovery Strategies and Agronomic Performance in Circular Farming: A Comprehensive Review. Nitrogen. 2025; 6(3):80. https://doi.org/10.3390/nitrogen6030080
Chicago/Turabian StyleShrivastava, Vaibhav, and Ikhlas Laasri. 2025. "Nutrient Recovery Strategies and Agronomic Performance in Circular Farming: A Comprehensive Review" Nitrogen 6, no. 3: 80. https://doi.org/10.3390/nitrogen6030080
APA StyleShrivastava, V., & Laasri, I. (2025). Nutrient Recovery Strategies and Agronomic Performance in Circular Farming: A Comprehensive Review. Nitrogen, 6(3), 80. https://doi.org/10.3390/nitrogen6030080







