Abstract
Nitrogen in all of its forms sustains Earth. In every known terrestrial and aquatic habitat, nitrogen controls microbial activity, plant productivity, trophic dynamics, and animal and human growth. This review has tried to show how nitrogen cycling is influenced by both terrestrial and marine ecosystems in addition to by changes spurred on by the climate. The availability, transformation, and final fate of nitrogen throughout the various ecosystems are influenced by these interconnected biochemical and biophysical processes, which are fueled by microbial communities. Predicting and reducing human impacts on the changing ecosystem requires an understanding of these complex interconnections. Anthropogenic and climatic changes alter the structure and function of soil microbial communities, as well as the main metabolic processes of the nitrogen cycle, such as nitrification, denitrification, nitrogen fixation, and ammonification. The mechanisms by which anthropogenic stress alters nitrogen cycling processes, the effects on ecosystem function, and possible mitigation techniques for a balanced nitrogen cycle are all discussed in this review.
1. Introduction
Nitrogen (N), one of the most essential for biological systems, is necessary for all life to persist [1]. All metabolic processes that result in the synthesis of proteins and the development of organs in humans, animals, and plants depend on nitrogen. In 3 oxidation states, nitrogen generates N-N bonds including N2 in conjunction with N0, hydrazine with N−II, and nitrous oxide (N2O) bonded to NI [2]. However, nitrogenases present in microbes have a fundamental ability to cleave the strong N-N bond. A carbon or oxygen seed is necessary for the stellar nucleosynthesis in the CNO cycle to create nitrogen [3], and the amount of N and its abundance will be determined based on the presence of CNO during planetary synthesis. Since NH3 is the main base in the troposphere of the Earth’s atmosphere and reduces the acidic gases produced by the oxidation of SO2 and NOx, 78% of molecular nitrogen is found [4]. Aerosols’ size, chemical makeup, and degree of neutralization are all influenced by NH3. In order to quantify reactive nitrogen species and trace gas fluxes at different spatial scales, atmospheric nitrogen is measured using satellite-based observations, box modelling, and leaf-level flux measurements [5]. Remarkably, trace levels of nitrates were found in Martian regolith by the Curiosity rover [6]. Titan shares a nitrogen-dominated atmosphere with Venus, and its atmosphere is rich in nitrogen (N2) [7]. As ammonia (NH3), it is also commonly found in comets and the gas giant planets of the solar system. The principal sources of naturally produced N are biological nitrogen fixation [8], wildfires [9], and lightning [10]. It is interesting to note that nitrogen is present in our surroundings as the gas dinitrogen. There are many recent changes in the nitrogen cycle [11,12], alterations in microbial diversity have resulted due to excess nitrogen fertilizer use, but this also opens the door to the discovery of sustainable methods, practices [13,14] and bacterial genes involved in the nitrogen cycle [15]. A study found a strong negative correlation between atmospheric nitrogen deposition and plant α-diversity, with a drop of 0.367 species for every kilogram increase in long-term N deposition per hectare, annually. High levels of atmospheric nitrogen deposition have been linked to a decline in plant species diversity [16]. In terms of total continuous radiative forcing, nitrogen cycling affects atmospheric concentrations of nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4) [17]. Microorganisms that fix nitrogen transform atmospheric nitrogen (N2) into forms that can be deployed, including ammonia (NH3). Ammonium (NH4+), which is widespread in soils, is absorbed by plant roots [18]. In situ spectroscopic detection techniques, flow injection analysis, ion-sensitive electrodes, etc., are used to quantify soil nitrogen [19].
Easily interconvertible form of nitrogen is nitrate (NO3−) especially in agricultural fields and water bodies. Urea, amino acids, and other compounds are examples of dissolved organic nitrogen (DON) in aquatic and forest systems. Although the process of biological N2H4 production is perhaps the least understood, it has been lately discovered that this molecule is relevant to primary metabolism [20]. The nitrogen cycle includes trace gases produced during nitrification and denitrification, such as nitrous oxide (N2O) and nitric oxide (NO). This review examines, briefly, the growing knowledge of how climate and ecosystems control nitrogen and their brief consequences biodiversity.
2. Ecosystems and Their Impacts on Nitrogen Dynamics
2.1. Forest Ecosystems
Different plant communities have a significant impact on nitrogen cycling in undisturbed soil ecosystems through microbial associations, soil organic matter, litter quantity and quality, and nitrogen mineralization rates. The ecological effects of human pressures like excessive fertilizer use and industrial pollutants are highlighted in Figure 1, which shows how nitrogen dynamics vary across healthy and disturbed ecosystems [21]. For example, grasslands with high root biomass promote steady nitrogen retention [22], while woods with high C:N litter promote delayed mineralization [23]. Additionally, tree growth is regulated and enhanced by nitrogen uptake by plant roots [24]. The annual net nitrogen mineralization (NNM) under pine has been demonstrated to be lower than that under birch and spruce, at roughly 12 kg N ha−1 yr−1 [25].
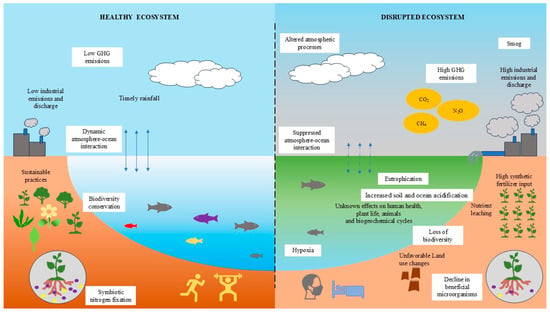
Figure 1.
Impact of ecosystems on nitrogen. This figure illustrates a comparative overview of a healthy versus a disrupted ecosystem, highlighting key environmental processes, anthropogenic activities, and their impacts on nitrogen. The left side represents a healthy ecosystem where sustainable agricultural practices and low industrial emissions maintain balanced nitrogen inputs, resulting in low greenhouse gases (GHG) levels such as nitrous oxide (N2O). These conditions promote dynamic atmosphere-ocean interactions, biodiversity conservation, and favorable conditions that symbiotic nitrogen fixation, efficient nitrification and denitrification processes, and overall ecological balance, underpinning ecological stability and human well-being. In contrast, the right side depicts a disrupted ecosystem, where excessive fertilizer application, land use changes, and industrial pollution elevate nitrogen inputs. This leads to nutrient leaching, eutrophication, hypoxia in aquatic systems, and increased emissions of reactive nitrogen species (e.g., N2O, NH3), contributing to smog and elevated greenhouse gases. These disturbances suppress atmosphere-ocean interactions, reduce microbial diversity, including nitrogen-transforming microbes and contribute to biodiversity loss and negative health outcomes in humans.
2.2. Terrestrial Agroecosystems
Rhizosphere activity, especially microbial activity that maintains the nitrogen cycle’s equilibrium, is influenced by a variety of exudates that are discharged by plant roots [26]. Legumes and the group of diazotrophic bacteria known as rhizobia (such as Rhizobium, Bradyrhizobium, and Azorhizobium spp.) have an endosymbiotic relationship that is orchestrated by a signaling network involving root exudates. Exudates including flavonoids catalyze the rhizobial nod genes expression in rhizobia involved in the soil nitrogen cycle [27]. Because organic acid exudates improve nitrogen mineralization and carbohydrate-rich exudates stimulate microbial nitrogen fixation, plants can access higher amounts of inorganic nitrogen [28]. Under natural agricultural practices like plant rotation and intercropping, leguminous plants facilitate biological nitrogen fixation, reducing the need for excessive amounts of synthetic fertilizer [29]. Modified litter inputs and climate stress also affect microbial activity and richness [30].
Reduced below-ground biodiversity [31], decreased nitrogen fixation [32], altered nitrogen and nitrogenous compounds, increased nitrification, and NO3− leaching all represent the outcomes of land use disturbances like agricultural conversion, deforestation, and urbanization that alter soil structure, organic matter, and microbial assemblages in terrestrial ecosystems [33]. The molecular alterations in ecosystems and climate physics responses that modify nitrogen cycle mechanisms, which in turn influence broader biological diversity, are less well understood. The overuse of synthetic N fertilizers to boost agricultural output worldwide has altered biodiversity (Table 1). Fast-growing species are promoted by N enrichment in aboveground biodiversity, which leads rare or N-efficient plants to be competitively removed. Climate change-induced N shifts influence the makeup of microbial communities, often favoring nitrophilous species. Nitrogen deposition encouraged the establishment of both common native species and invasive species in monoculture settings [34]. When N dynamics are disturbed, it affects ecosystem services like pollination [35], pest control [36], and carbon storage [37]. Changes in plant N have an effect on populations of herbivores and decomposers [38].

Table 1.
Effects of enhanced nitrogen on biodiversity (Based on studies from 1873 to 2022).
Negative anthropogenic factors include monoculture in agriculture along with excess application of synthetic N chemicals, use of machinery made of harmful synthetic material, deforestation, non-natural farming, etc., which impact BNF [14]. Inorganic nitrogen reduces symbiotic nitrogen fixation by blocking NRAMP2-mediated iron transport within soybean nodules, which is essential for appropriate nodule activity [49]. By artificially inflating the NH4+ and NO3− pools, this alters the rates of nitrification and denitrification and increases N2O emissions.
Ammonification is impacted by disruptions to microbial habitat and substrate availability [50]. Low pH from acid rain or ammonium-containing fertilizers, particularly in nitrifiers, impairs microbial enzymatic activity [51]. Salinity stress can inhibit BNF and denitrification by altering the osmotic balance and community composition of soil microorganisms [52,53]. By disrupting microbial metabolism and diversity, toxic substances reduce all nitrogen processes, especially nitrification and fixation [54]. Overuse of sewage sludge can accelerate ammonification and alter nitrogen pathways in anaerobic pockets towards DNRA. Similarly, increase in carbon may alter the abundance of denitrifying and nitrifying microorganisms.
2.3. Marine and Ocean Ecosystems
The nitrogen cycle processes, which in turn influence primary output and carbon dioxide uptake, are greatly influenced by marine ecosystems, which also play a crucial role in regulating the global N cycle [55]. The sequestration and storage of massive amounts of fixed nitrogen in the form of dissolved organic nitrogen (DON), one of the planet’s greatest fixed nitrogen reserves with roughly 70 Pg, is one of the most significant roles of marine ecosystems [56]. Importantly, recent research indicates that refractory DON is dominated by 15N-depleted heterocyclic-N structures, as opposed to the previously recognized amide-dominated high molecular weight (HMW) material [57].
The continuous transformation of nitrogen between its various forms has been rendered possible by an array of microbiological processes in marine ecosystems, some of which are analogous to the nitrogen cycle on land. By transforming atmospheric N2 into accessible ammonia (NH3), diazotrophs, for instance, conduct biological nitrogen fixation (BNF) in oceans, which supplies an additional nitrogen to marine systems [58]. In addition to warm, oligotrophic surface waters, its range is far more extensive than previously thought, encompassing hypoxic basins, oxygen minimum zones (OMZs), the deep sea, estuaries, and coral reefs. Intricate nitrogen cycle interactions are coordinated by the diverse and metabolically adaptive microbial community in conjunction with the dominant physical and chemical ocean conditions. Although the exact roles of some of these elements are still unknown, factors including light, oxygen, phosphorus, temperature, and iron have a significant impact on BNF rates and the distribution of diazotrophs [59,60]. Ocean nitrification requires the right balance of ammonia-oxidizing bacteria and archaea to catalyze the transformation of NH3 to nitrite (NO2−), and subsequently nitrite-oxidizing bacteria to utilize NO2− to form nitrate (NO3−) [61]. Denitrifying bacteria, the ocean’s main sink for fixed nitrogen, transform NO3− back into N2. Their primary habitats are sub-oxic water columns, such as OMZs, and marine sediments which are dependent on organic matter [62]. Anaerobic ammonium oxidation (Anammox) transforms ammonium (NH4+) and NO2− to N2 in the absence of oxygen and is a N depreciating process, notably in OMZs and sediments [61]. Dissimilatory nitrate reduction to ammonia (DNRA) is an anaerobic process that primarily occurs in anoxic environments such as sediments and OMZs and converts NO3− to NH4+. Nitrate/nitrite-dependent anaerobic methane oxidation, or N-damo, has been discovered as a process of N-loss that links the carbon and nitrogen cycles, but currently less understood [63]. The amount, form, and fate of nitrogen in marine habitats is determined by interdependent processes that are powered by marine microbial communities. They significantly affect the overall health of marine ecosystems as well as the cycling of nutrients.
Anthropogenic activities significantly disturb the typical marine N cycle [64]. Fossil fuel burning, human sewage, and agricultural runoff all contribute to nutrient pollution, which causes toxic algal blooms and oxygen deprivation, ultimately contributing to the loss of biodiversity [65,66]. Climate change can accelerate ocean acidification, increase river runoff, deoxygenate the atmosphere, and influence nitrification, all of which can worsen these effects [62]. Nitrogen loss, particularly in low-oxygen regions such as the Bay of Bengal, the Gulf of Guinea, and the region near the Amazon River mouth, where more denitrification paradoxically lowers primary production when high N supply is used [62,67]. Spectrophotometry, colorimetry, fluorometry, and other techniques are used to measure nitrogen species in aquatic systems, including total dissolved nitrogen (TDN), NO3−, and NH3. High-resolution monitoring is increasingly being performed using emerging technologies as remote sensing and microfluidic sensors [68].
Understanding these intricate relationships is necessary to forecast and lessen human impacts on the changing ocean. The atmosphere and its aerosol composition play a significant role in the marine nitrogen cycle as a source of fixed nitrogen to the oceans and as a medium for complex changes that affect marine biogeochemistry, productivity, and climate.
Anthropogenic influences include activities notably traffic and industry emissions, agricultural practices including the use of nitrogen-rich fertilizers, human waste, and farm animal feces. Agriculture, if conducted unsustainably, emits 93% ammonia. Reactive nitrogen molecules are released into the atmosphere, where they undergo a variety of complex chemical and photochemical reactions before being reintroduced into ecosystems (either wet or dry) through nitrogen deposition [69,70]. Reactive nitrogen plays a major role in atmospheric aerosol formation [71]. Aerosol catalysis requires gaseous ammonia to be absorbed and neutralized with nitric or sulfuric acids. The atmospheric concentration of NOx, mostly from industry and traffic, has a substantial impact on the generation of nitric oxide, which is dependent on the presence of ozone (O3) and specific radicals. Furthermore, these radicals are essential for the oxidation of SO2 and NO2 as well as the synthesis of peroxyacetylnitrate (PAN), a secondary pollutant in photochemical smog. Nitrogen wet deposition, which can damage already vulnerable ecosystems, relies upon cloud interactions. Chemical processes occurring inside cloud droplets and generate nitrogen-containing inorganic (such as ammonium nitrate) and organic aerosol particles influence air quality. Furthermore, nitrogen pollution can alter the properties of clouds, perhaps producing more numerous, smaller, more reflecting droplets that might lower the climate. Atmospheric organic nitrogen warrants future investigations as this may harbor important roles in atmospheric N cycle. Nitrogen increase in ocean surface may slow down BNF rates according to some models [66]. This is because non-nitrogen-fixing phytoplankton may become more competitive when nitrogen is more abundant. For instance, temperature-driven glacier meltwater may provide iron and nutrients, creating a favorable habitat for diazotrophs and helping to fix nitrogen in Arctic coastal waters [72].
3. Climate Effects on Nitrogen Cycling
Climate factors such as temperature, precipitation, pH, and CO2 levels impact nitrogen transformation processes, which in turn impact the related enzymatic pathways and biochemical reactions (Table 2).

Table 2.
Impact of climate on major nitrogen reactions.
3.1. Increased Temperatures
Warmer temperatures can alter the amount of nitrogen available in soils and water bodies by accelerating microbial processes such as nitrification (converting ammonium to nitrate), denitrification (reducing nitrate to gases), and mineralization. Temperature variations can either promote or inhibit biological nitrogen fixing by particular bacteria and cyanobacteria, which in turn affects the conversion of atmospheric nitrogen (N2) to ammonium (NH4+). For instance, strains of Azospirillum brasilense may fix nitrogen at temperatures as high as 42 °C [84], but the marine diazotroph Trichodesmium exhibits optimal development and nitrogen fixation between 24 °C and 30 °C, with considerable inhibition occurring at temperatures over 34 °C [85]. As temperatures rise, the microbial breakdown of organic nitrogen into ammonium (more specifically, mineralization or ammonification) accelerates the rate at which nitrogen becomes available in the soil [86]. The aerobic oxidation of ammonium (NH4+) to nitrate (NO3−) often enhances nitrification rates up to an optimum temperature; after that, activity may decline. Ammonia-oxidizing archaea exhibit optimal activity and growth between 30 °C and 37 °C, whereas ammonia-oxidizing bacteria thrive in the narrower temperature range of 16 °C to 23 °C [87]. Higher temperatures have been demonstrated to enhance earthworm activity, which significantly affects the microorganisms that cycle nitrogen in the soil. This involves promoting nitrogen cycle processes such as mineralization, denitrification, and plant absorption and increasing the amount of the amoA gene associated with ammonia-oxidizing bacteria (AOB) [88]. Furthermore, heat exacerbates nitrogen losses in earthworm-inhabited arable soils, particularly in systems with significant organic inputs, by increasing the likelihood of nitrate leaching and nitrous oxide (N2O) emissions [89]. High temperatures may accelerate denitrification rates, which may contribute to higher emissions of nitrogen gases like nitrous oxide (N2O), a potent greenhouse gas, especially in wet or anaerobic soil conditions [90]. Nitrogen loss to the atmosphere can originate from increased ammonia (NH3) volatilization from soils brought on by warmer temperatures [91]. Cyanobacterial and phytoplankton blooms spurred by rising temperatures significantly alter nitrogen cycle and worsen eutrophication in aquatic ecosystems. In habitats where abundant phytoplankton exist may influence phosphorus which affects nitrogen fixation. However, systems dominated by slow-growing phytoplankton with high ratios of nitrogen to phosphate consumption worsen nitrogen deficits and promote nitrogen fixation [92]. Rising water temperatures and marine heatwaves have been shown to reduce the number of Rotifers, while increasing the number of larger omnivorous Copepods [93]. By altering nitrifier activity and suppressing nitrification processes, this alteration weakens top-down regulation, allowing unrestrained phytoplankton growth, and increasing biomass, it impacts nitrate dynamics in ecosystems [94]. The dynamics of nitrogen input in marine ecosystems are significantly altered by warmer temperatures, which raise the biomass levels of cyanobacterial species in the pelagic environment [95]. Anammox bacteria have been demonstrated to be inhibited by warming, which may cause increased nitrogen flow towards denitrification and the subsequent production of N2O [82]. In conclusion, because rising temperatures accelerate nitrogen cycle activities, alter nitrogen availability, and increase emissions of nitrogenous gases, they have an impact on ecosystem function and climate feedback globally. The changes in nitrogen cycling brought about by climate change and their ripple effects on ecosystems and human health are summarized in Figure 2 (see the health section).
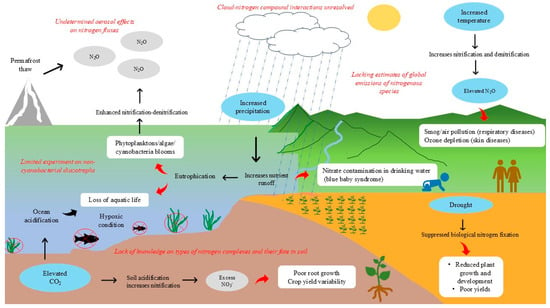
Figure 2.
Impact of climate change on nitrogen and its consequences on biological systems. Climate change may negatively hamper nitrogen cycling leading to environmental concerns and sustainable planetary development. Rising temperatures accelerate nitrogen transformations, resulting in increased emissions of nitrous oxide (N2O), a potent greenhouse gas that contributes to smog and air pollution, exacerbating respiratory health issues, ozone reduction in stratosphere, and human skin problem. Prolonged rainfall causes nitrate to leak into water bodies from excess synthetic N during farming leading to poor health. Drought conditions hinder biological nitrogen fixation (BNF), reducing plant growth and resulting in lower crop yields. Elevated CO2 levels contribute to soil acidification, enhancing nitrification and leading to an excess of nitrates in the soil. This negatively affects plant health by impairing root development and causing variability in crop yields. Nutrient leaching into water bodies triggers eutrophication and promotes the excessive growth of phytoplankton and cyanobacteria. When coupled with ocean acidification caused by elevated CO2 levels, these processes drive hypoxia and the loss of aquatic biodiversity. Several critical uncertainties and knowledge gaps continue to limit our understanding of nitrogen dynamics under changing climatic conditions (as highlighted in red, italics).
3.2. Unpredictable Rainfall
Increased precipitation raises the complexity and connectedness of arbuscular mycorrhizal fungal (AMF) networks through modifications to soil pH, which increases their ability to nitrify and denitrify [96]. Warmer soil conditions caused by increased precipitation can enhance the releases of nitrogen gases, including nitrous oxide (N2O), and create anaerobic conditions that promote denitrification [97]. Changes in soil moisture have an effect on microbial activity as optimal moisture levels encourage nitrogen mineralization; nevertheless excessive or insufficient water may limit microbial activity and impact nitrogen availability [98]. Strong winds and heavy rains can erode topsoil, removing nitrogen-rich organic matter and reducing soil fertility. Between the 1990s and 2010, the overall soil fertility in Northeast China decreased by 4.5%, leading to a general decline in soil health, according to [99]. Reduced precipitation or drought, on the other hand, can slow the nitrogen cycle by decreasing microbial activity and plant nitrogen uptake, which leads to nitrogen accumulation in soils. This is evident in grasslands and shrublands, where nitrification and mineralization either decrease or stay constant during dry conditions [100]. By reducing nitrogen mineralization and the transformation of nitrogen into compounds that plants can utilize, climate conditions can significantly restrict plant development. By lowering nitrogen losses from leaching and denitrification, dry conditions can momentarily preserve nitrogen in the soil. However, when the moisture returns, the nitrogen cycle resumes. During a longer dry season caused by decreased precipitation, soil NO3− concentrations increased by 25–64%, and mineralization and nitrification rates increased by 25–40%. Nitrogen declines due to excessive NO3− leaching when prolonged precipitation occurs [101]. Water pollution and increased soil nitrogen loss could result from changes in rainfall patterns’ effects on runoff and nitrogen leaching. Increased rainfall intensity can expedite the leaching of nitrogen, especially nitrate, from soils into groundwater [102]. This results in eutrophication in aquatic settings and a reduction in the quantity of nitrogen available in the soil [103]. Erratic and unpredictable cause alterations in concentrations N during atmospheric deposition. Dust discharge can alter marine bacterial populations and enhance N2 fixation by supplying active bacteria to the ocean surface and fostering viable microorganisms such diazotrophs [104,105]. Sporulating microorganisms during aerial transport germinate quickly when deposited in water bodies with higher nutrient concentrations [106] and this may be of concern if the populations of pathogenic microorganisms are higher [107]. Furthermore, dust inputs encourage phytoplankton development [108], which indirectly increases nitrogen demand and accelerates the nitrogen cycle. Moreover, increased ammonium (NHx) and nitrate (NOᵧ) reduce methanotrophy (methane oxidation) and long-term atmospheric N deposition caused by human activities reduces biological nitrogen fixation (BNF) in peatlands [109]. Because methanotrophic bacteria help these systems provide nitrogen-fixing microorganisms, this has an impact on BNF [110].
3.3. Elevated CO2
Growing plants are often stimulated by elevated CO2, which can increase their intake and need for nitrogen [111]. This could lead to changes in the availability of nitrogen in the soil. Plants have a higher requirement of N when high CO2 ensue [112]. Increased CO2 can have an impact on soil microbial populations and related processes, such as nitrification and nitrogen mineralization, albeit the impacts can differ depending on the ecosystem and soil conditions. In other cases, the increased plant biomass caused by higher CO2 may be constrained by the availability of nitrogen [113]. Because increased CO2 suppresses the growth and activity of autotrophic bacteria and archaea involved in nitrification, inorganic nitrogen stores (NH4) in lowland soil systems have decreased [114]. An increase in CO2 supports certain plant development, but the dilution of tissue N concentration alters metabolic pathways [115]. as seen in a study where legume N content decreased to ~2 mg g−1 when CO2 (400 ppm initially) concentration rose to 1200 ppm [116]. Furthermore, by changing C:N inputs to the soil, an increase in CO2 indirectly alters microbial activity, which may reduce the availability of soil nitrate and change the structure of plant communities.
Because nitrogenase efficacy decreases at elevated pCO2 levels above 400 μatm, Trichodesmium erythraeum development and N2 fixation rates are reduced in aquatic conditions [117]. Elevated CO2 causes ocean acidification, which changes the grazing pattern of microzooplanktons, increasing the growth of phytoplankton species and influencing nitrogen fixation and N loss rates [118]. Ocean acidification additionally results in increased rates of denitrification and N2O emissions from eutrophic waters.
4. Nutrient Imbalances and Its Impact on Nitrogen Cycle
Nutrient surpluses, primarily from the improper use of synthetic fertilizers, have caused significant nitrogen losses in terrestrial systems (Figure 1). Overfertilization raises the availability of ammonium, which encourages nitrification and denitrification and results in large gaseous nitrogen losses as N2O and N2. A long-term examination from 1970 to 2020 found that up to 45% of surplus nitrogen is lost by volatilization, 50% through denitrification, and 46% through nitrate leaching [119]. Irrigation trials showed that nitrification was enhanced by irrigation with higher salt levels (8 g L−1), while it was reduced by irrigation with moderately saline water (4 g L−1) as opposed to irrigation with groundwater (1 g L−1). Additionally, as salinity levels increased, denitrification gene expression increased likewise, showing how salt-induced nutrient imbalances can alter the balance and efficiency of nitrogen cycling processes [120]. The nitrogen cycle routes in aquatic environments can be significantly altered by nutrient imbalance. The Mississippi River’s nutrient runoff produces hypoxic zones in the Gulf of Mexico, where nitrification-denitrification dominates nitrogen loss and anammox has a negligible impact [121]. Ref. [122] found that hypoxia altered the benthic nitrogen cycle by increasing DNRA recycling and reducing nitrogen loss through anammox and denitrification, which is different from trends observed in the Gulf of Mexico. Nutrient imbalances caused by eutrophication, especially fluctuating nitrate (NO3−) levels, have a major effect on nitrogen cycling in aquatic sediments. The presence of macroalgae significantly boosts the production of nitrous oxide (N2O), dissimilatory nitrate reduction to ammonium (DNRA), and nitrate-ammonifying bacteria including Shewanella and Arcobacter when nitrate levels are low [123]. However, at high nitrate concentrations, denitrification represents the main mechanism for removing nitrogen, and DNRA and N2O emissions sharply decrease.
4.1. Effect on Human Health
Overfertilization accelerates nitrification [124], leading to elevated NO3− levels in crops, particularly in green vegetables like spinach and chard [125]. These NO3− can transform into NO2− when consumed, which can subsequently produce NOCs, which are carcinogenic compounds linked to an increased risk to humans [126]. Literature on how soil health affects human health has been summarized [11,127]. Nitrogen oxides have been implicated in mortality from respiratory diseases, according to [128].
4.2. Effect on Plants and Soil Habitats
A study [129], states that high nitrogen levels, particularly those of ammonium, can result in osmotic stress, rice photosynthetic inhibition, and oxidative damage to plant tissues.
Furthermore, elevated nitrogen levels hinder the synthesis of secondary metabolites necessary for plant defense, making crops more vulnerable to pests and illnesses [130]. Acidification of the soil due to ammonia re-deposition could eventually affect crop yields [131]. Because soil acidification inhibits key nitrogen-cycling microorganisms including ammonia-oxidizing bacteria (AOB) and complete ammonia oxidizers (Comammox), there is a decrease in nitrification activity. Acidified soils not only lower emissions of nitrous oxide (N2O), but they also limit the amount of substrate available and suppress the expression of denitrification genes (nirS, nirK), which limits the natural denitrification pathway that is necessary for nitrogen removal [132]. Vegetation declines significantly reduced soil total nitrogen (TN), ammonium (NH4+-N), and microbiological nitrogen while increasing nitrate (NO3−-N) content. The soil becomes more aerobic as vegetation undergoes decomposition, which promotes nitrifying bacteria to convert more NH4+ to NO3− [133]. Naturally occurring diazotrophs [134] and soil degradation that reduces carbon availability limit BNF [135].
PVC microplastics significantly raised the soil’s NH4+-N content and decreased the NO3−-N content by up to 91% (reduced nitrification), causing the soil microbiota to change into a microbial system with more nitrogen-fixing microorganisms which was estimated through gene abundance data [136].
The negative correlations between NO3−-N concentration and nitrification activity, in addition to the decrease in the archael taxon (Candidatus Nitrosocosmicus) and the rise in bacterial populations (e.g., Amycolatopsis, Sinomonas, Nocardia, Bradyrhizobium, and Burkholderia), provided evidence for the microbial shift away from nitrification pathways. Increases in fungi like Exophiala and Cladophialophora may be dangerous to both humans and vertebrates.
In a microcosm experiment using salt marsh sediment treated with microplastics consisting of polyethylene (PE), polyvinyl chloride (PVC), polyurethane foam (PUF), or polylactic acid (PLA), it was discovered that the makeup of the soil microbial population and nitrogen cycling mechanisms had changed [137]. In contrast to the control sediments free of microplastic, PUF and PLA-amended sediments promote nitrification and denitrification, whereas PVC amendment inhibits both processes. According to these results, different types of microplastics may significantly affect the nitrogen cycle processes in sediments by serving as organic carbon substrates for microbial populations NO2– and NO3– were highest in the 16-day PUF and PLA treatments, whereas NH4+ was lowest (high bacterial amoA gene and nirS implying high rates of nitrification and denitrification). High NH4+ was the outcome of PVC treatment; decreased denitrification activity and nitrification inhibition were indicated by the lowest abundance of the amoA and nirS genes.
5. Conclusions
The nitrogen cycle may be impacted by biodiversity, which is impacted by climate change and other human-caused changes to ecosystems. In ecosystems, particularly in agricultural and natural biological systems, nitrogen cycling is a complicated but essential process. Between soil, plants, microbes, and the atmosphere, nitrogen is absorbed, changed, and moved. As was previously mentioned, several nitrogen cycle reactions, including accumulation in some habitats and depletion in others, may be influenced by human activities and climate change triggers. The transport and balance of nitrogen in ecosystems are generally altered by climate change, which has an impact on water quality, plant development, and soil fertility. Future research must fill in the knowledge inadequacies in our existing understanding of nitrogen and its different forms.
5.1. Policy and Mitigation
Since increasing nitrogen pollution could have detrimental effects on biodiversity and climate change, policy and scientific management solutions are being pursued. Numerous principles, implementation frameworks, and concrete objectives for sustainable development and nitrogen have been compiled and published [138]. Reducing soil air and water contaminants that contribute to the disturbance of nitrogen compounds and cycles may be the goal of national and international initiatives. The nitrogen cycle is directly impacted by the growing integration of nutrient recovery and circular economy concepts into India’s waste management framework. Anaerobic digestion of organic residues and livestock waste is encouraged by the Swachh Bharat Mission (Urban and Gramin) and the Solid Waste Management Rules (2016). Complementary programs like GOBARdhan and the Waste-to-Energy/National Bioenergy Programme produce nitrogen-rich digestate and slurry that replace synthetic fertilizers and generate renewable energy. Additionally, by decreasing the waste of organic matter and excessive usage of fertilizer, NITI Aayog directly addresses nitrogen inefficiencies by promoting the closure of nutrient loops and the valorization of agricultural and urban leftovers. The UN Sustainable Development Goals, which focus on reducing nutrient pollution, creating sustainable urban systems, and reducing greenhouse gas emissions linked to nitrogen, are closely aligned with these actions. By promoting compost, green manures, and biofertilizers that substitute biologically fixed and recycled organic sources for mineral nitrogen, India’s Paramparagat Krishi Vikas Yojana (PKVY) encourages cluster-based organic farming. Similarly to this, Zero-Budget Natural Farming (ZBNF) encourages the use of microbial formulations and soil-restorative techniques like mulching and intercropping, which improve soil microbial activity, increase nitrogen fixation, and reduce runoff and volatilization. Although yield results differ by crop and location, there is evidence that these systems can significantly lower the costs of nitrogen inputs and environmental leakage. When taken as a whole, these programs represent a shift from a linear nitrogen-use model, which is characterized by significant losses and reliance on fertilizers, to a circular model, which values waste, conserves nitrogen, and lessens environmental effects.
Policy initiatives across many countries are being carried out and await positive outcomes. There is a special aim of the UN to reduce nitrogen waste by 2030 awaiting its outcome. Sewage regulation, the employing of agricultural fertilizers, wastewater management, agroforestry, conserving water bodies free of algae blooms, and the adoption of natural farming for healthy soil management are some of the measures depicted in Figure 3.
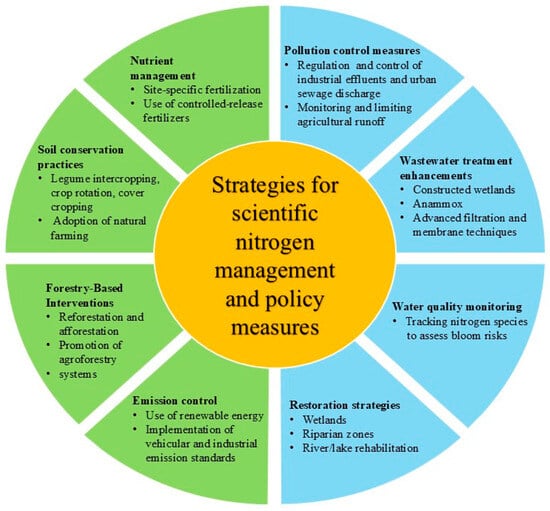
Figure 3.
Strategies for terrestrial and aquatic nitrogen management. This diagram outlines holistic strategies for nitrogen management across terrestrial and aquatic ecosystems.
5.2. Future Directions
Further scientific investigation of the biochemistry of the nitrogen cycle in relation to natural ecosystems is necessary. Measurements of different forms of nitrogen are not available spatially and temporally across continents and these may be monitored in future. To minimize the trade-offs between carbon dioxide, methane, and nitrous oxide, a deeper comprehension of the effects of various decarbonization policies and measures, such as the use of alternate fuel for marine shipping, incentives to produce bioenergy, and soil carbon sequestration initiatives is required. It is essential to control the nitrogen and carbon cycles in concert. Encourage nature-based farming solutions, clean water, and efficient bioremediation of natural water bodies. Support international targets, such as the UN’s “halve nitrogen waste” to generate benefits for the entire world. The relationship between the marine DON, its components, and cycling is unclear, and it is unclear how microbial roles may contribute to its cycling. The effects of climate change on specific nitrogen cycle responses are still poorly known and must be considered considering biodiversity impacts. Despite the complexity of the nitrogen cycle, scientists and policymakers may collaborate to create more efficient methods for regulating nitrogen levels to support sustainability and environmental health by filling in these knowledge gaps and investigating potential avenues for future research.
Author Contributions
Conceptualization, R.M.; methodology, R.M.; software, R.M. and S.B.M.; validation, R.M. and S.B.M.; formal analysis, R.M.; investigation, R.M.; resources, R.M.; data curation, R.M., S.B.M. and N.H.; writing—original draft preparation, R.M. and S.B.M.; writing—review and editing, R.M., S.B.M. and N.H.; visualization, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank our team members for useful discussions and inputs.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cleaves, H.J. Nitrogen. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–3. [Google Scholar]
- Ferousi, C.; Majer, S.H.; DiMucci, I.M.; Lancaster, K.M. Biological and Bioinspired Inorganic N–N Bond-Forming Reactions. Chem. Rev. 2020, 120, 5252–5307. [Google Scholar] [CrossRef]
- Molla, M.; Vilchez, J.M.; Gavilan, M.; Diaz, A.I. The Nitrogen-to-Oxygen Evolution in Galaxies: The Role of the Star Formation Rate. Mon. Not. R. Astron. Soc. 2006, 372, 1069–1080. [Google Scholar] [CrossRef]
- Altieri, K.E.; Hastings, M.G.; Peters, A.J.; Oleynik, S.; Sigman, D.M. Isotopic Evidence for a Marine Ammonium Source in Rainwater at Bermuda. Glob. Biogeochem. Cycles 2014, 28, 1066–1080. [Google Scholar] [CrossRef]
- Delaria, E.R.; Cohen, R.C. Measurements of Atmosphere–Biosphere Exchange of Oxidized Nitrogen and Implications for the Chemistry of Atmospheric NOx. Acc. Chem. Res. 2023, 56, 1720–1730. [Google Scholar] [CrossRef]
- Duri, L.G.; Caporale, A.G.; Rouphael, Y.; Vingiani, S.; Palladino, M.; De Pascale, S.; Adamo, P. The Potential for Lunar and Martian Regolith Simulants to Sustain Plant Growth: A Multidisciplinary Overview. Front. Astron. Space Sci. 2022, 8, 747821. [Google Scholar] [CrossRef]
- Nixon, C.A. The Composition and Chemistry of Titan’s Atmosphere. ACS Earth Space Chem. 2024, 8, 406–456. [Google Scholar] [CrossRef] [PubMed]
- Threatt, S.D.; Rees, D.C. Biological Nitrogen Fixation in Theory, Practice, and Reality: A Perspective on the Molybdenum Nitrogenase System. FEBS Lett. 2023, 597, 45–58. [Google Scholar] [CrossRef]
- Lindaas, J.; Pollack, I.B.; Garofalo, L.A.; Pothier, M.A.; Farmer, D.K.; Kreidenweis, S.M.; Campos, T.L.; Flocke, F.; Weinheimer, A.J.; Montzka, D.D.; et al. Emissions of Reactive Nitrogen From Western U.S. Wildfires During Summer 2018. J. Geophys. Res. Atmos. 2021, 126, e2020JD032657. [Google Scholar] [CrossRef]
- Schumann, U.; Huntrieser, H. The Global Lightning-Induced Nitrogen Oxides Source. Atmos. Chem. Phys. 2007, 7, 3823–3907. [Google Scholar] [CrossRef]
- Mattoo, R.; Suman, B.M. Microbial Roles in the Terrestrial and Aquatic Nitrogen Cycle—Implications in Climate Change. FEMS Microbiol. Lett. 2023, 370, fnad061. [Google Scholar] [CrossRef]
- Fields, S. Global Nitrogen: Cycling out of Control. Environ. Health Perspect. 2004, 112, A556–A563. [Google Scholar] [CrossRef]
- Mattoo, R.; Suman, B.M. Comparison of Rhizospheric Functional Diversity Between Chemically Fertilized and Bioinoculated Millet. In Millet Rhizosphere. Rhizosphere Biology; Pudake, R.N., Kumari, M., Sapkal, D.R., Sharma, A.K., Eds.; Springer: Singapore, 2023; pp. 149–170. [Google Scholar]
- Mattoo, R.; Gowda, M. Harnessing Soil Bacteria and Their Benefits for Sustainable Agriculture with Changing Climate. CABI Rev. 2022. [Google Scholar] [CrossRef]
- Denkhaus, L.; Siffert, F.; Einsle, O. An Unusual Active Site Architecture in Cytochrome c Nitrite Reductase NrfA-1 from Geobacter Metallireducens. FEMS Microbiol. Lett. 2023, 370, fnad068. [Google Scholar] [CrossRef]
- van der Plas, F.; Hautier, Y.; Ceulemans, T.; Alard, D.; Bobbink, R.; Diekmann, M.; Dise, N.B.; Dorland, E.; Dupré, C.; Gowing, D.; et al. Atmospheric Nitrogen Deposition Is Related to Plant Biodiversity Loss at Multiple Spatial Scales. Glob. Change Biol. 2024, 30, e17445. [Google Scholar] [CrossRef] [PubMed]
- Suddick, E.C.; Whitney, P.; Townsend, A.R.; Davidson, E.A. The Role of Nitrogen in Climate Change and the Impacts of Nitrogen–Climate Interactions in the United States: Foreword to Thematic Issue. Biogeochemistry 2013, 114, 1–10. [Google Scholar] [CrossRef]
- Gurung, A.; Mattoo, R. Microbes in Biogeochemical Cycles: A Perspective on Climate Resilient Agriculture. CABI Rev. 2021, 16, 6. [Google Scholar] [CrossRef]
- Liu, J.; Cai, H.; Chen, S.; Pi, J.; Zhao, L. A Review on Soil Nitrogen Sensing Technologies: Challenges, Progress and Perspectives. Agriculture 2023, 13, 743. [Google Scholar] [CrossRef]
- Kartal, B.; Maalcke, W.J.; de Almeida, N.M.; Cirpus, I.; Gloerich, J.; Geerts, W.; Op den Camp, H.J.M.; Harhangi, H.R.; Janssen-Megens, E.M.; Francoijs, K.-J.; et al. Molecular Mechanism of Anaerobic Ammonium Oxidation. Nature 2011, 479, 127–130. [Google Scholar] [CrossRef]
- Mattoo, R.; Mallikarjuna, S. Soil Microbiome Influences Human Health In the Context of Climate Change. Future Microbiol. 2023, 18, 845–859. [Google Scholar] [CrossRef]
- Lama, S.; Velescu, A.; Leimer, S.; Weigelt, A.; Chen, H.; Eisenhauer, N.; Scheu, S.; Oelmann, Y.; Wilcke, W. Plant Diversity Influenced Gross Nitrogen Mineralization, Microbial Ammonium Consumption and Gross Inorganic N Immobilization in a Grassland Experiment. Oecologia 2020, 193, 731–748. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A.; Schultz, J.C. Nitrogen Cycling in a Northern Hardwood Forest: Do Species Matter? Biogeochemistry 2004, 67, 289–308. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Y.; Eissenstat, D.M.; He, C.; Sheng, L. Belowground Responses of Woody Plants to Nitrogen Addition in a Phosphorus-Rich Region of Northeast China. Trees 2020, 34, 143–154. [Google Scholar] [CrossRef]
- Becker, H.; Aosaar, J.; Varik, M.; Morozov, G.; Aun, K.; Mander, Ü.; Soosaar, K.; Uri, V. Annual Net Nitrogen Mineralization and Litter Flux in Well-Drained Downy Birch, Norway Spruce and Scots Pine Forest Ecosystems. Silva Fenn. 2018, 52, 10013. [Google Scholar] [CrossRef]
- Zhai, X.; Zheng, Y.; Ma, F.; Ren, L.; Bai, W.; Tian, Q.; Zhang, W.-H. Root Exudation Is Involved in Regulation of Nitrogen Transformation under Mowing in a Temperate Steppe. Soil. Biol. Biochem. 2024, 195, 109481. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How Plant Root Exudates Shape the Nitrogen Cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Liu, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Root Exudates Shift How N Mineralization and N Fixation Contribute to the Plant-Available N Supply in Low Fertility Soils. Soil. Biol. Biochem. 2022, 165, 108541. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.-W.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, Z.; Diao, Z.; Zhang, Y.; Wang, Y.; Ma, L. The Effects of Litter Input and Increased Precipitation on Soil Microbial Communities in a Temperate Grassland. Front. Microbiol. 2024, 15, 1347016. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Chen, J.; Shen, R.F. Land-Use Change Has a Greater Effect on Soil Diazotrophic Community Structure than the Plant Rhizosphere in Acidic Ferralsols in Southern China. Plant Soil. 2021, 462, 445–458. [Google Scholar] [CrossRef]
- Bomfim, B.; Silva, L.C.R.; Doane, T.A.; Horwath, W.R. Interactive Effects of Land-Use Change and Topography on Asymbiotic Nitrogen Fixation in the Brazilian Atlantic Forest. Biogeochemistry 2019, 142, 137–153. [Google Scholar] [CrossRef]
- Ren, M.; Li, C.; Gao, X.; Niu, H.; Cai, Y.; Wen, H.; Yang, M.; Siddique, K.H.M.; Zhao, X. High Nutrients Surplus Led to Deep Soil Nitrate Accumulation and Acidification after Cropland Conversion to Apple Orchards on the Loess Plateau, China. Agric. Ecosyst. Environ. 2023, 351, 108482. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, X.; Chen, Y.; Liu, L.; Li, M.; Wang, T.; Sun, Y.; Li, H.; Guo, X. Nitrogen Deposition Enhances the Competitive Advantage of Invasive Plant Species over Common Native Species through Improved Resource Acquisition and Absorption. Ecol. Process. 2024, 13, 61. [Google Scholar] [CrossRef]
- David, T.I.; Storkey, J.; Stevens, C.J. Understanding How Changing Soil Nitrogen Affects Plant–Pollinator Interactions. Arthropod Plant Interact. 2019, 13, 671–684. [Google Scholar] [CrossRef]
- Li, Z.; Xu, B.; Du, T.; Ma, Y.; Tian, X.; Wang, F.; Wang, W. Excessive Nitrogen Fertilization Favors the Colonization, Survival, and Development of Sogatella Furcifera via Bottom-Up Effects. Plants 2021, 10, 875. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of Forest Soil Respiration in Response to Nitrogen Deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Bakker, E.S.; Knops, J.M.H.; Milchunas, D.G.; Ritchie, M.E.; Olff, H. Cross-site Comparison of Herbivore Impact on Nitrogen Availability in Grasslands: The Role of Plant Nitrogen Concentration. Oikos 2009, 118, 1613–1622. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, M.; Zhang, J.; Liu, Z.; Mo, J. Soil Diazotrophs Sustain Nitrogen Fixation under High Nitrogen Enrichment via Adjustment of Community Composition. mSystems 2024, 9, e00547-24. [Google Scholar] [CrossRef]
- Dai, Y.; Di, H.J.; Cameron, K.C.; He, J.-Z. Effects of Nitrogen Application Rate and a Nitrification Inhibitor Dicyandiamide on Ammonia Oxidizers and N2O Emissions in a Grazed Pasture Soil. Sci. Total Environ. 2013, 465, 125–135. [Google Scholar] [CrossRef]
- Yue, P.; Zuo, X.; Li, K.; Cui, X.; Wang, S.; Misselbrook, T.; Liu, X. The Driving Effect of Nitrogen-Related Functional Microorganisms under Water and Nitrogen Addition on N2O Emission in a Temperate Desert. Sci. Total Environ. 2021, 772, 145470. [Google Scholar] [CrossRef]
- Yang, X.; Tang, S.; Ni, K.; Shi, Y.; Yi, X.; Ma, Q.; Cai, Y.; Ma, L.; Ruan, J. Long-Term Nitrogen Addition Increases Denitrification Potential and Functional Gene Abundance and Changes Denitrifying Communities in Acidic Tea Plantation Soil. Environ. Res. 2023, 216, 114679. [Google Scholar] [CrossRef]
- Blakemore, R. Critical Decline of Earthworms from Organic Origins under Intensive, Humic SOM-Depleting Agriculture. Soil. Syst. 2018, 2, 33. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Tie, L.; Liu, X.; Liu, X.; Zhao, A.; Lai, J.; Xiao, L.; You, C.; Huang, C. Effects of Nitrogen Addition on Soil Faunal Abundance: A Global Meta-analysis. Glob. Ecol. Biogeogr. 2022, 31, 1655–1666. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wu, Y.; Yu, Y.; Sun, J.; Mao, D.; Zhang, G. Intensified Effect of Nitrogen Forms on Dominant Phytoplankton Species Succession by Climate Change. Water Res. 2024, 264, 122214. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, K.; Du, Y.; Li, K.; Liu, Z.; Jeppesen, E.; Søndergaard, M. Increased Nitrogen Loading Boosts Summer Phytoplankton Growth by Alterations in Resource and Zooplankton Control: A Mesocosm Study. Front. Environ. Sci. 2021, 9, 772314. [Google Scholar] [CrossRef]
- Zhao, R.; Hannisdal, B.; Mogollon, J.M.; Jørgensen, S.L. Nitrifier Abundance and Diversity Peak at Deep Redox Transition Zones. Sci. Rep. 2019, 9, 8633. [Google Scholar] [CrossRef]
- Zhou, J.; Mogollón, J.M.; van Bodegom, P.M.; Barbarossa, V.; Beusen, A.H.W.; Scherer, L. Effects of Nitrogen Emissions on Fish Species Richness across the World’s Freshwater Ecoregions. Environ. Sci. Technol. 2023, 57, 8347–8354. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Yao, X.-L.; Zhang, J.; Liu, S.; Cao, H.-R.; Bai, S.; Chen, C.-Q.; Zhang, D.-X.; Xu, A.; et al. Inorganic Nitrogen Inhibits Symbiotic Nitrogen Fixation through Blocking NRAMP2-Mediated Iron Delivery in Soybean Nodules. Nat. Commun. 2024, 15, 8946. [Google Scholar] [CrossRef]
- Isobe, K.; Ise, Y.; Kato, H.; Oda, T.; Vincenot, C.E.; Koba, K.; Tateno, R.; Senoo, K.; Ohte, N. Consequences of Microbial Diversity in Forest Nitrogen Cycling: Diverse Ammonifiers and Specialized Ammonia Oxidizers. ISME J. 2020, 14, 12–25. [Google Scholar] [CrossRef]
- Tong, D.; Xu, R. Effects of Urea and (NH4)2SO4 on Nitrification and Acidification of Ultisols from Southern China. J. Environ. Sci. 2012, 24, 682–689. [Google Scholar] [CrossRef]
- Kayasth, M.; Gera, R.; Dudeja, S.S.; Sharma, P.K.; Kumar, V. Studies on Salinization in Haryana Soils on Free-living Nitrogen-fixing Bacterial Populations and Their Activity. J. Basic. Microbiol. 2014, 54, 170–179. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wan, W.; Luo, X.; Zheng, L.; He, G.; Huang, D.; Chen, W.; Huang, Q. High Salinity Inhibits Soil Bacterial Community Mediating Nitrogen Cycling. Appl. Environ. Microbiol. 2021, 87, e01366-21. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Oon, Y.-S.; Oon, Y.-L.; Khan, K.; Deng, M.; Li, L.; Song, K.; Jiang, X.; Xia, Z. Microplastics Transport and Impact on Nitrogen Cycling and N2O Emissions in Estuaries. Environ. Pollut. 2025, 383, 126869. [Google Scholar] [CrossRef] [PubMed]
- Broek, T.A.B.; McCarthy, M.D.; Ianiri, H.L.; Vaughn, J.S.; Mason, H.E.; Knapp, A.N. Dominant Heterocyclic Composition of Dissolved Organic Nitrogen in the Ocean: A New Paradigm for Cycling and Persistence. Proc. Natl. Acad. Sci. USA 2023, 120, e2305763120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ward, B.B.; Sigman, D.M. Global Nitrogen Cycle: Critical Enzymes, Organisms, and Processes for Nitrogen Budgets and Dynamics. Chem. Rev. 2020, 120, 5308–5351. [Google Scholar] [CrossRef]
- McCarthy, M.; Hedges, J.; Benner, R. Major Biochemical Composition of Dissolved High Molecular Weight Organic Matter in Seawater. Mar. Chem. 1996, 55, 281–297. [Google Scholar] [CrossRef]
- Rabalais, N.N. Nitrogen in Aquatic Ecosystems. AMBIO J. Hum. Environ. 2002, 31, 102–112. [Google Scholar] [CrossRef]
- Pajares, S.; Ramos, R. Processes and Microorganisms Involved in the Marine Nitrogen Cycle: Knowledge and Gaps. Front. Mar. Sci. 2019, 6, 739. [Google Scholar] [CrossRef]
- Arrigo, K.R. Marine Microorganisms and Global Nutrient Cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef]
- Lam, P.; Kuypers, M.M.M. Microbial Nitrogen Cycling Processes in Oxygen Minimum Zones. Annu. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef]
- Tivig, M.; Keller, D.P.; Oschlies, A. Riverine Nitrogen Supply to the Global Ocean and Its Limited Impact on Global Marine Primary Production: A Feedback Study Using an Earth System Model. Biogeosciences 2021, 18, 5327–5350. [Google Scholar] [CrossRef]
- Raghoebarsing, A.A.; Pol, A.; van de Pas-Schoonen, K.T.; Smolders, A.J.P.; Ettwig, K.F.; Rijpstra, W.I.C.; Schouten, S.; Damsté, J.S.S.; Op den Camp, H.J.M.; Jetten, M.S.M.; et al. A Microbial Consortium Couples Anaerobic Methane Oxidation to Denitrification. Nature 2006, 440, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Duce, R.A.; LaRoche, J.; Altieri, K.; Arrigo, K.R.; Baker, A.R.; Capone, D.G.; Cornell, S.; Dentener, F.; Galloway, J.; Ganeshram, R.S.; et al. Impacts of Atmospheric Anthropogenic Nitrogen on the Open Ocean. Science 2008, 320, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of Hypoxia for Marine Biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef]
- Jickells, T.D.; Buitenhuis, E.; Altieri, K.; Baker, A.R.; Capone, D.; Duce, R.A.; Dentener, F.; Fennel, K.; Kanakidou, M.; LaRoche, J.; et al. A Reevaluation of the Magnitude and Impacts of Anthropogenic Atmospheric Nitrogen Inputs on the Ocean. Glob. Biogeochem. Cycles 2017, 31, 289–305. [Google Scholar] [CrossRef]
- Landolfi, A.; Dietze, H.; Koeve, W.; Oschlies, A. Overlooked Runaway Feedback in the Marine Nitrogen Cycle: The Vicious Cycle. Biogeosciences 2013, 10, 1351–1363. [Google Scholar] [CrossRef]
- Siriwardana, H.; Samarasekara, R.S.M.; Anthony, D.; Vithanage, M. Measurements and Analysis of Nitrogen and Phosphorus in Oceans: Practice, Frontiers, and Insights. Heliyon 2024, 10, e28182. [Google Scholar] [CrossRef]
- Allaerts, W. On Nitrogen, Anthropogenic Aerosols, Farmland and Biodiversity Estimation. Austin Environ. Sci. 2022, 7, 1086. [Google Scholar] [CrossRef]
- Delbaere, B.; Whitfield, C.; Evans, D. Impact of Atmospheric Nitrogen Deposition on Biodiversity; European Environment Agency: Copenhagen, Denmark, 2014.
- Li, X.; Maring, H.; Savoie, D.; Voss, K.; Prospero, J.M. Dominance of Mineral Dust in Aerosol Light-Scattering in the North Atlantic Trade Winds. Nature 1996, 380, 416–419. [Google Scholar] [CrossRef]
- Schlangen, I.; Leon-Palmero, E.; Moser, A.; Xu, P.; Laursen, E.; Löscher, C.R. Nitrogen Fixation in Arctic Coastal Waters (Qeqertarsuaq, West Greenland): Influence of Glacial Melt on Diazotrophs, Nutrient Availability, and Seasonal Blooms. EGUsphere, 2024; preprint. [Google Scholar] [CrossRef]
- Liang, J.; Qi, X.; Souza, L.; Luo, Y. Processes Regulating Progressive Nitrogen Limitation under Elevated Carbon Dioxide: A Meta-Analysis. Biogeosciences 2016, 13, 2689–2699. [Google Scholar] [CrossRef]
- Liao, H.; Zheng, C.; Li, J.; Long, J.; Li, Y.; Yao, H. Factors Influencing the Global Distribution of Soil Free-Living Nitrogen Fixation in Terrestrial Ecosystems. Appl. Soil. Ecol. 2025, 213, 106285. [Google Scholar] [CrossRef]
- Fu, Y.; Ren, X.; Zhu, B. Responses of Soil Nitrogen Transformation and N2O Emission to Soil PH and Hydrothermal Changes. Agronomy 2025, 15, 1005. [Google Scholar] [CrossRef]
- Zhao, Y.; Luan, J.-W.; Wang, Y.; Yang, H.; Liu, S.-R. Effects of Simulated Drought and Phosphorus Addition on Nitrogen Mineralization in Tropical Lowland Rain Forests. Chin. J. Plant Ecol. 2022, 46, 102–113. [Google Scholar] [CrossRef]
- Mao, C.; Wang, Y.; Ran, J.; Wang, C.; Yang, Z.; Yang, Y. Effects of Warming and Precipitation Change on Soil Nitrogen Cycles: A Meta-Analysis. J. Plant Ecol. 2025, 18, rtaf051. [Google Scholar] [CrossRef]
- Le, T.T.H.; Fettig, J.; Meon, G. Kinetics and Simulation of Nitrification at Various PH Values of a Polluted River in the Tropics. Ecohydrol. Hydrobiol. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, P.; Yuan, S.; Wang, K.; Wang, S.; Jiang, X. Role of Nitrogen Cycling Functional Genes and Their Key Influencing Factors in Eutrophic Aquatic Ecosystems. Environ. Rev. 2025, 33, 1–10. [Google Scholar] [CrossRef]
- Wan, R.; Chen, Y.; Zheng, X.; Su, Y.; Li, M. Effect of CO2 on Microbial Denitrification via Inhibiting Electron Transport and Consumption. Environ. Sci. Technol. 2016, 50, 9915–9922. [Google Scholar] [CrossRef]
- Liu, B.; Mørkved, P.T.; Frostegård, Å.; Bakken, L.R. Denitrification Gene Pools, Transcription and Kinetics of NO, N2O and N2 Production as Affected by Soil PH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef]
- Tan, E.; Zou, W.; Zheng, Z.; Yan, X.; Du, M.; Hsu, T.-C.; Tian, L.; Middelburg, J.J.; Trull, T.W.; Kao, S. Warming Stimulates Sediment Denitrification at the Expense of Anaerobic Ammonium Oxidation. Nat. Clim. Change 2020, 10, 349–355. [Google Scholar] [CrossRef]
- Xie, H.; Ji, D.; Zang, L. Effects of Inhibition Conditions on Anammox Process. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 012149. [Google Scholar] [CrossRef]
- Rai, R. High-Temperature-Adapted Azospirillum brasilense Strains: Growth and Interaction Response on Associative Nitrogen Fixation, Mineral Uptake and Yield of Cheena ( Panicum miliaceum L.) Genotypes in Calcareous Soil. J. Agric. Sci. 1988, 110, 321–329. [Google Scholar] [CrossRef]
- Breitbarth, E.; Oschlies, A.; LaRoche, J. Physiological Constraints on the Global Distribution of Trichodesmium—Effect of Temperature on Diazotrophy. Biogeosciences 2007, 4, 53–61. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wang, D.; Ma, J.; Xue, K.; An, Z.; Luo, W.; Sheng, Y. Effects of Temperature and Humidity on Soil Gross Nitrogen Transformation in a Typical Shrub Ecosystem in Yanshan Mountain and Hilly Region. Life 2023, 13, 643. [Google Scholar] [CrossRef]
- Taylor, A.E.; Giguere, A.T.; Zoebelein, C.M.; Myrold, D.D.; Bottomley, P.J. Modeling of Soil Nitrification Responses to Temperature Reveals Thermodynamic Differences between Ammonia-Oxidizing Activity of Archaea and Bacteria. ISME J. 2017, 11, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Wang, C.; Liu, X.; Liu, M. Earthworm Regulation of Nitrogen Pools and Dynamics and Marker Genes of Nitrogen Cycling: A Meta-Analysis. Pedosphere 2022, 32, 131–139. [Google Scholar] [CrossRef]
- Marhan, S.; Auber, J.; Poll, C. Additive Effects of Earthworms, Nitrogen-Rich Litter and Elevated Soil Temperature on N2O Emission and Nitrate Leaching from an Arable Soil. Appl. Soil. Ecol. 2015, 86, 55–61. [Google Scholar] [CrossRef]
- Tan, X.; Shao, D.; Gu, W. Effects of Temperature and Soil Moisture on Gross Nitrification and Denitrification Rates of a Chinese Lowland Paddy Field Soil. Paddy Water Environ. 2018, 16, 687–698. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Wan, Y.; Li, Y.; Cai, W.; Guo, C.; Qin, X.; Song, C.; Wilkes, A. Air Warming and CO2 Enrichment Cause More Ammonia Volatilization from Rice Paddies: An OTC Field Study. Sci. Total Environ. 2021, 752, 142071. [Google Scholar] [CrossRef]
- Mills, M.M.; Arrigo, K.R. Magnitude of Oceanic Nitrogen Fixation Influenced by the Nutrient Uptake Ratio of Phytoplankton. Nat. Geosci. 2010, 3, 412–416. [Google Scholar] [CrossRef]
- Huỳnh, T.; Horváth, Z.; Pálffy, K.; Kardos, V.; Szabó, B.; Dobosy, P.; Vad, C.F. Heatwave-induced Functional Shifts in Zooplankton Communities Result in Weaker Top-down Control on Phytoplankton. Ecol. Evol. 2024, 14, e70096. [Google Scholar] [CrossRef]
- Wan, X.S.; Sheng, H.; Dai, M.; Church, M.J.; Zou, W.; Li, X.; Hutchins, D.A.; Ward, B.B.; Kao, S. Phytoplankton-Nitrifier Interactions Control the Geographic Distribution of Nitrite in the Upper Ocean. Glob. Biogeochem. Cycles 2021, 35, e2021GB007072. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Zhang, H.; Chaguaceda, F.; Geng, H.; Hansson, L. Climate Warming and Heat Waves Alter Harmful Cyanobacterial Blooms along the Benthic–Pelagic Interface. Ecology 2020, 101, e03025. [Google Scholar] [CrossRef] [PubMed]
- Mushinski, R.M.; Payne, Z.C.; Raff, J.D.; Craig, M.E.; Pusede, S.E.; Rusch, D.B.; White, J.R.; Phillips, R.P. Nitrogen Cycling Microbiomes Are Structured by Plant Mycorrhizal Associations with Consequences for Nitrogen Oxide Fluxes in Forests. Glob. Change Biol. 2021, 27, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, A.A.; Hondzo, M.; Kozarek, J.L.; Staley, C.; Wang, P.; Lurndahl, N.; Sadowsky, M.J. Intermittent Flooding of Organic-rich Soil Promotes the Formation of Denitrification Hot Moments and Hot Spots. Ecosphere 2019, 10, e02549. [Google Scholar] [CrossRef]
- Guntiñas, M.E.; Leirós, M.C.; Trasar-Cepeda, C.; Gil-Sotres, F. Effects of Moisture and Temperature on Net Soil Nitrogen Mineralization: A Laboratory Study. Eur. J. Soil. Biol. 2012, 48, 73–80. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, H.; Wang, X.; Ma, R.; Lin, C. Response of Soil Fertility to Soil Erosion on a Regional Scale: A Case Study of Northeast China. J. Clean. Prod. 2024, 434, 140360. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.-G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought Effects on Soil Carbon and Nitrogen Dynamics in Global Natural Ecosystems. Earth Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Chen, J.; Kuzyakov, Y.; Jenerette, G.D.; Xiao, G.; Liu, W.; Wang, Z.; Shen, W. Intensified Precipitation Seasonality Reduces Soil Inorganic N Content in a Subtropical Forest: Greater Contribution of Leaching Loss Than N2O Emissions. J. Geophys. Res. Biogeosci 2019, 124, 494–508. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, W.; Wang, S.; Ma, L.; Min, L.; Shen, Y. Variation of Nitrate Sources Affected by Precipitation with Different Intensities in Groundwater in the Piedmont Plain Area of Alluvial-Pluvial Fan. J. Environ. Manag. 2024, 367, 121885. [Google Scholar] [CrossRef]
- Han, H.; Xiao, R.; Gao, G.; Yin, B.; Liang, S.; lv, X. Influence of a Heavy Rainfall Event on Nutrients and Phytoplankton Dynamics in a Well-Mixed Semi-Enclosed Bay. J. Hydrol. 2023, 617, 128932. [Google Scholar] [CrossRef]
- Rahav, E.; Ovadia, G.; Paytan, A.; Herut, B. Contribution of Airborne Microbes to Bacterial Production and N2 Fixation in Seawater upon Aerosol Deposition. Geophys. Res. Lett. 2016, 43, 719–727. [Google Scholar] [CrossRef]
- Na, H.; Qi, J.; Zhen, Y.; Yao, X.; Gao, H. Asian Dust-Transported Bacteria Survive in Seawater and Alter the Community Structures of Coastal Bacterioplankton in the Yellow Sea. Glob. Planet. Change 2023, 224, 104115. [Google Scholar] [CrossRef]
- Genitsaris, S.; Stefanidou, N.; Beeri-Shlevin, Y.; Viner-Mozzini, Y.; Moustaka-Gouni, M.; Ninio, S.; Sukenik, A. Air-Dispersed Aquatic Microorganisms Show Establishment and Growth Preferences in Different Freshwater Colonisation Habitats. FEMS Microbiol. Ecol. 2021, 97, fiab122. [Google Scholar] [CrossRef]
- Mattoo, R. Targeting Emerging Mycobacterium avium Infections: Perspectives into Pathways and Antimicrobials for Future Interventions. Future Microbiol. 2021, 16, 753–764. [Google Scholar] [CrossRef]
- Meng, X.; Yao, F.; Zhang, J.; Liu, Q.; Liu, Q.; Shi, L.; Zhang, D. Impact of Dust Deposition on Phytoplankton Biomass in the Northwestern Pacific: A Long-Term Study from 1998 to 2020. Sci. Total Environ. 2022, 813, 152536. [Google Scholar] [CrossRef]
- Saiz, E.; Sgouridis, F.; Drijfhout, F.P.; Peichl, M.; Nilsson, M.B.; Ullah, S. Chronic Atmospheric Reactive Nitrogen Deposition Suppresses Biological Nitrogen Fixation in Peatlands. Environ. Sci. Technol. 2021, 55, 1310–1318. [Google Scholar] [CrossRef]
- Larmola, T.; Leppänen, S.M.; Tuittila, E.-S.; Aarva, M.; Merilä, P.; Fritze, H.; Tiirola, M. Methanotrophy Induces Nitrogen Fixation during Peatland Development. Proc. Natl. Acad. Sci. USA 2014, 111, 734–739. [Google Scholar] [CrossRef]
- Dong, X.; Lin, H.; Wang, F.; Shi, S.; Sharifi, S.; Wang, S.; Ma, J.; He, X. Elevated CO2 and Nitrogen Supply Boost N Use Efficiency and Wheat (T. aestivum cv. Yunmai) Growth and Differentiate Soil Microbial Communities Related to Ammonia Oxidization. Plants 2024, 13, 2345. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 Effects on Plant Carbon, Nitrogen, and Water Relations: Six Important Lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.; Ellsworth, D.S.; West, J.B.; Tilman, D.; Knops, J.M.H.; Naeem, S.; Trost, J. Nitrogen Limitation Constrains Sustainability of Ecosystem Response to CO2. Nature 2006, 440, 922–925. [Google Scholar] [CrossRef]
- Zhang, K.; Lei, W.; Zhang, H.; Xu, C.; Xiao, J.; Li, S.; Liang, M.; He, J.; Lai, Y.; Li, R.; et al. Inhibition of Autotrophic Nitrifiers in a Nitrogen-Rich Paddy Soil by Elevated CO2. Nat. Geosci. 2024, 17, 1254–1260. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why Are Nitrogen Concentrations in Plant Tissues Lower under Elevated CO2? A Critical Examination of the Hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, F.; Hao, L.; Yu, J.; Guo, L.; Zhou, H.; Ma, C.; Zhang, X.; Xu, M. Elevated CO2 Concentration Induces Photosynthetic Down-Regulation with Changes in Leaf Structure, Non-Structural Carbohydrates and Nitrogen Content of Soybean. BMC Plant Biol. 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Shen, R.; Zhang, F.; Wen, Z.; Chang, S.; Lin, W.; Kranz, S.A.; Luo, Y.-W.; Kao, S.-J.; Morel, F.M.M.; et al. The Complex Effects of Ocean Acidification on the Prominent N2–Fixing Cyanobacterium Trichodesmium. Science 2017, 356, 527–531. [Google Scholar] [CrossRef]
- Shi, W.; Fu, X.; Han, Y.; Qin, J.; Sun, J. Impact of Ocean Acidification on Microzooplankton Grazing Dynamics. Front. Mar. Sci. 2024, 11, 1414932. [Google Scholar] [CrossRef]
- Elsayed, H.; Beusen, A.; Prusty, A.K.; Bouwman, L. Long-Term Variations (1970–2020) and Spatial Patterns of Nitrogen and Phosphorus Soil Budgets and Fates in Indian Agriculture. Nutr. Cycl. Agroecosyst. 2025, 130, 17–32. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, G.; Han, Q.; Zhang, J.; Dang, H.; Ning, H.; Gao, Y.; Sun, J. Long-Term Saline Water Irrigation Affected Soil Carbon and Nitrogen Cycling Functional Profiles in the Cotton Field. Front. Microbiol. 2024, 15, 1310387. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Newell, S.E.; Carini, S.A.; Gardner, W.S. Denitrification Dominates Sediment Nitrogen Removal and Is Enhanced by Bottom-Water Hypoxia in the Northern Gulf of Mexico. Estuaries Coasts 2015, 38, 2279–2294. [Google Scholar] [CrossRef]
- Song, G.; Liu, S.; Zhang, J.; Zhu, Z.; Zhang, G.; Marchant, H.K.; Kuypers, M.M.M.; Lavik, G. Response of Benthic Nitrogen Cycling to Estuarine Hypoxia. Limnol. Oceanogr. 2021, 66, 652–666. [Google Scholar] [CrossRef]
- Wong, W.W.; Greening, C.; Shelley, G.; Lappan, R.; Leung, P.M.; Kessler, A.; Winfrey, B.; Poh, S.C.; Cook, P. Effects of Drift Algae Accumulation and Nitrate Loading on Nitrogen Cycling in a Eutrophic Coastal Sediment. Sci. Total Environ. 2021, 790, 147749. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Ji, L.; Ma, L.; Ruan, J. Heavy Nitrogen Application Increases Soil Nitrification through Ammonia-Oxidizing Bacteria Rather than Archaea in Acidic Tea (Camellia sinensis L.) Plantation Soil. Sci. Total Environ. 2020, 717, 137248. [Google Scholar] [CrossRef]
- Calderón, R.; Albornoz, F.; Jara, C.; Palma, P.; Arancibia-Miranda, N.; Manquián-Cerda, K.; Herrera, C.; Urrutia, J.; Gamboa, C.; Karthikraj, R.; et al. Exploring the Uptake, Accumulation, and Distribution of Nitrate in Swiss Chard and Spinach and Their Impact on Food Safety and Human Health. Food Chem. 2025, 467, 142345. [Google Scholar] [CrossRef] [PubMed]
- Atakisi, E.; Merhan, O. Nitric Oxide Synthase and Nitric Oxide Involvement in Different Toxicities. In Nitric Oxide Synthase—Simple Enzyme-Complex Roles; InTech: London, UK, 2017. [Google Scholar]
- Mattoo, R.; Nagaraju, U. Why Should We Worry about Soil Health? Curr. Sci. 2025, 128, 431–432. [Google Scholar]
- César, A.C.G.; Carvalho, J.A., Jr.; Nascimento, L.F.C. Association between NOx Exposure and Deaths Caused by Respiratory Diseases in a Medium-Sized Brazilian City. Braz. J. Med. Biol. Res. 2015, 48, 1130–1135. [Google Scholar] [CrossRef]
- Yang, S.; Hao, D.; Jin, M.; Li, Y.; Liu, Z.; Huang, Y.; Chen, T.; Su, Y. Internal Ammonium Excess Induces ROS-Mediated Reactions and Causes Carbon Scarcity in Rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef]
- Wang, Y.; Di, B.; Sun, Z.; Sonali; Donovan-Mak, M.; Chen, Z.; Wang, M. Multi-Omics and Physiological Analysis Reveal Crosstalk Between Aphid Resistance and Nitrogen Fertilization in Wheat. Plant Cell Environ. 2025, 48, 2024–2039. [Google Scholar] [CrossRef]
- Kuttippurath, J.; Singh, A.; Dash, S.P.; Mallick, N.; Clerbaux, C.; Van Damme, M.; Clarisse, L.; Coheur, P.-F.; Raj, S.; Abbhishek, K.; et al. Record High Levels of Atmospheric Ammonia over India: Spatial and Temporal Analyses. Sci. Total Environ. 2020, 740, 139986. [Google Scholar] [CrossRef]
- Meng, C.; Xing, Y.; Ding, Y.; Zhang, Q.; Di, H.; Tang, C.; Xu, J.; Li, Y. Soil Acidification Induced Variation of Nitrifiers and Denitrifiers Modulates N2O Emissions in Paddy Fields. Sci. Total Environ. 2023, 882, 163623. [Google Scholar] [CrossRef]
- He, W.; Ma, W.; Du, J.; Chang, W.; Li, G. Effect of Vegetation Degradation on Soil Nitrogen Components and N-Cycling Enzyme Activities in a Wet Meadow on the Qinghai–Tibetan Plateau. Plants 2025, 14, 1549. [Google Scholar] [CrossRef]
- Xu, M.; Fan, L.; Li, A.; Liu, Q.; Yu, G.; Wang, S.; Zhang, B.; Ye, Q.; Mo, J.; Zheng, M. Plant and Microbial Carbon Are Important Drivers of Free-Living Nitrogen Fixation in Tropical Forest Soils: A New Discovery of Carbon-Driven Nitrogen Input. Geophys. Res. Lett. 2024, 51, e2024GL111238. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, H.; Liu, J.; Yao, Y.; Wang, X.; Rong, G.; Zhao, X.; Shao, M.; Wei, X. Soil Erosion Significantly Reduces Organic Carbon and Nitrogen Mineralization in a Simulated Experiment. Agric. Ecosyst. Environ. 2021, 307, 107232. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, Y.; Doyle, E.; Zhu, C.; Jin, X.; Chen, Z.; Wang, C.; He, H.; Zhou, D.; Gu, C. Microplastics Altered Soil Microbiome and Nitrogen Cycling: The Role of Phthalate Plasticizer. J. Hazard. Mater. 2022, 427, 127944. [Google Scholar] [CrossRef]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics Affect Sedimentary Microbial Communities and Nitrogen Cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Kanter, D.R.; Lassaletta, L.; Móring, A.; Raghuram, N.; Read, N. The Nitrogen Decade: Mobilizing Global Action on Nitrogen to 2030 and Beyond. One Earth 2021, 4, 10–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).