Fertilization Promotes the Recovery of Plant Productivity but Decreases Biodiversity in a Khorchin Degraded Grassland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Design of Experiments
2.3. Sample Collection and Testing
2.4. Data Analysis
3. Results
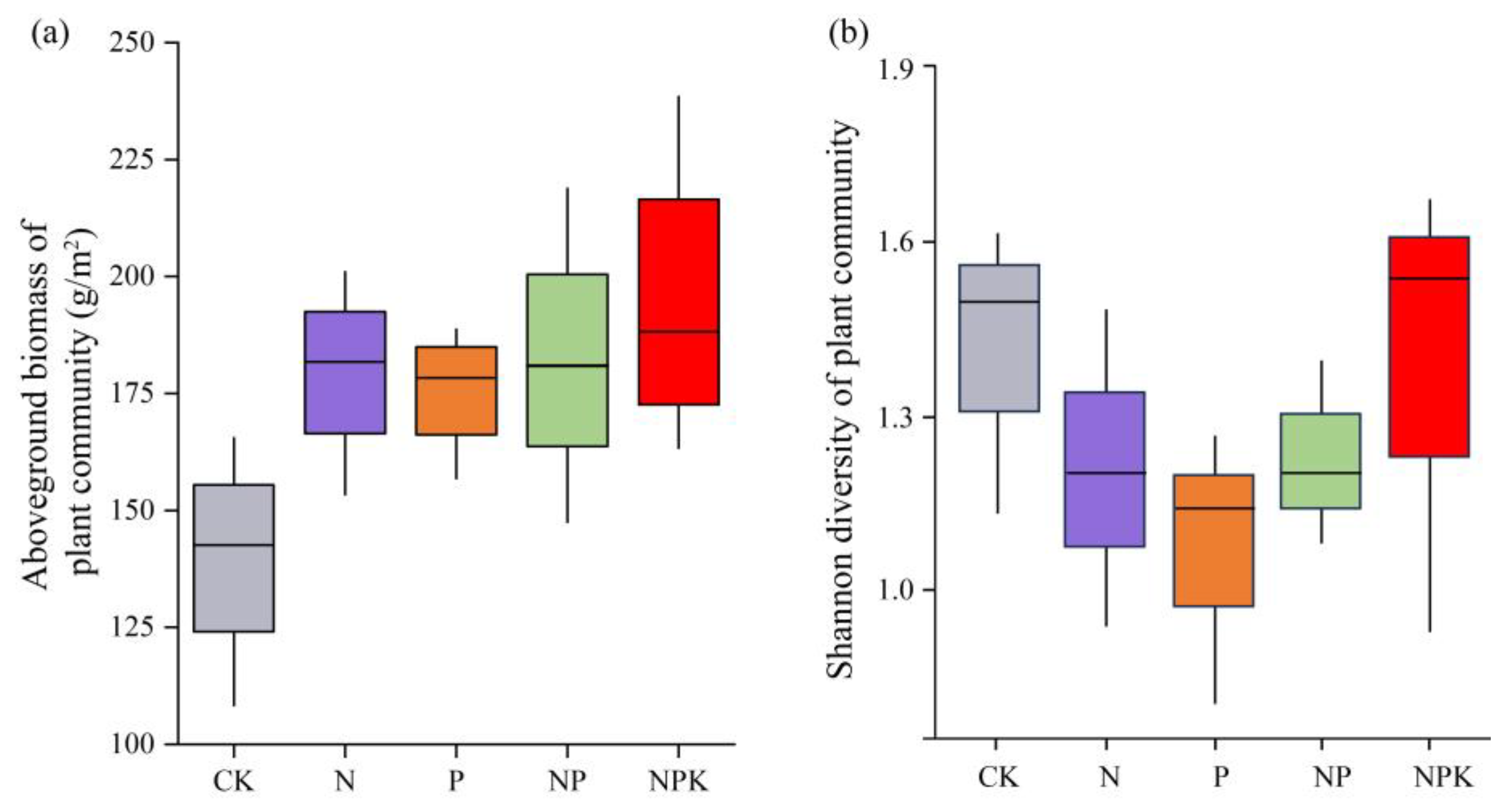
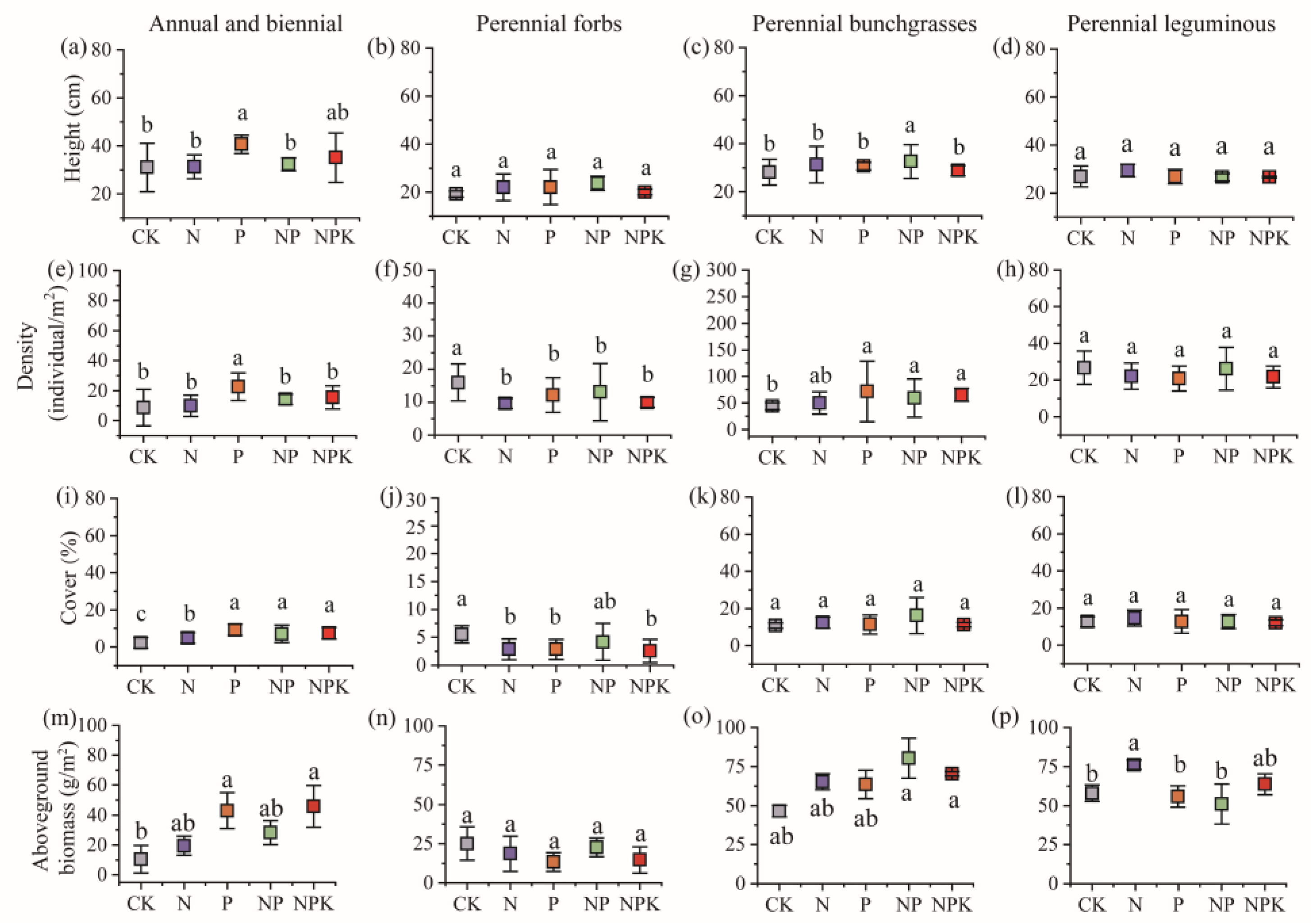
3.1. Effect of Different Fertilization Treatments on the Plant Community
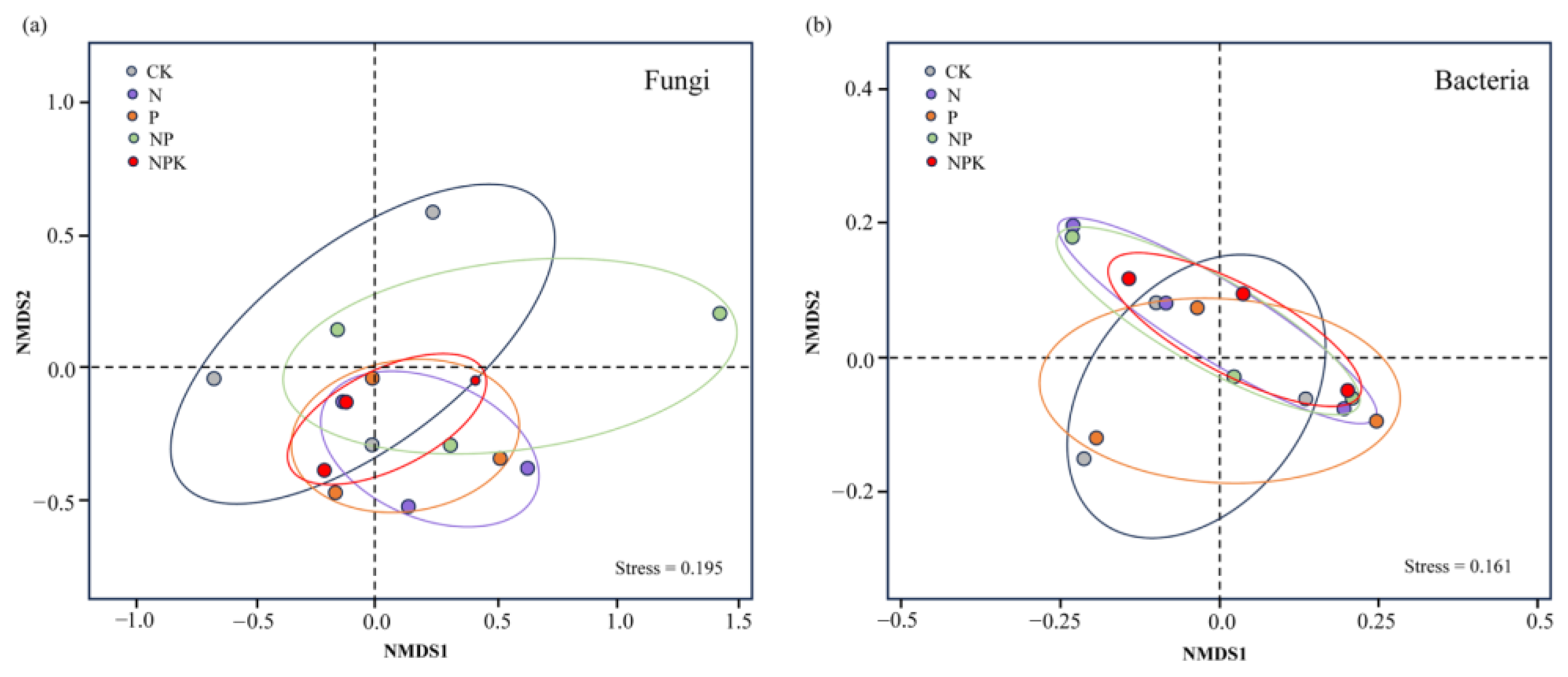
3.2. Effect of Different Fertilization Treatments on the Microbial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fay, P.A.; Prober, S.M.; Harpole, W.S.; Knops, J.M.H.; Bakker, J.D.; Borer, E.T.; Lind, E.M.; MacDougall, A.S.; Seabloom, E.W.; Wragg, P.D.; et al. Grassland productivity limited by multiple nutrients. Nat. Plants 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Chen, Q.; Dittert, K.; Taube, F.; Li, S. Nitrogen, phosphorus and potassium nutritional status of semiarid steppe grassland in Inner Mongolia. Plant Soil 2011, 340, 265–278. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.; Zheng, L.; Zhou, T.; Cong, N.; Hou, G.; Song, M.; Tian, J.; Zhang, X.; Zhu, J. Restoration effects of fertilization and grazing exclusion on different degraded alpine grasslands: Evidence from a 10-year experiment. Ecol. Eng. 2021, 170, 106361. [Google Scholar] [CrossRef]
- Fry, E.L.; Manning, P.; Macdonald, C.; Hasegawa, S.; De Palma, A.; Power, S.A.; Singh, B.K. Shifts in microbial communities do not explain the response of grassland ecosystem function to plant functional composition and rainfall change. Soil Biol. Biochem. 2016, 92, 199–210. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, M.; Xing, Q.; Liu, Y.; Wang, B. Comparative Study on Grassland Degradation in Zhalute Banner Based on Two Surveys. Grassl. Prataculture 2022, 34, 11–17. [Google Scholar]
- Ren, Y.; Zhang, B.; Chen, X. Desertification Sensitivity Assessment in Horqin Sandy Land. J. Desert Res. 2023, 43, 159–169. [Google Scholar]
- Liu, C.; Li, H.; Liu, K.; Shao, X.; Huang, J.; Siri, M.; Feng, C.; Yang, X. Vegetation Characteristics of the Main Grassland Types in China Respond Differently to the Duration of Enclosure: A Meta-Analysis. Agronomy 2023, 13, 854. [Google Scholar] [CrossRef]
- Wang, M.-M.; Liu, X.-P.; He, Y.-H.; Zhang, T.-H.; Wei, J.; Chelmge; Sun, S.-S. How enclosure influences restored plant community changes of different initial types in Horqin Sandy Land. Chin. J. Plant Ecol. 2019, 43, 672–684. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Cleland, E.; Harpole, W. Nitrogen enrichment and plant communities. Ann. N. Y. Acad. Sci. 2010, 1195, 46–61. [Google Scholar] [CrossRef]
- Simkin, S.M.; Allen, E.B.; Bowman, W.D.; Clark, C.M.; Belnap, J.; Brooks, M.L.; Cade, B.S.; Collins, S.L.; Geiser, L.H.; Gilliam, F.S.; et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 4086–4091. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for Light Causes Plant Biodiversity Loss After Eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.Z.; Bouwman, A.F.; Martinez Rodríguez, R.; Beusen, A.H.W.; van Ittersum, M.K. Negative global phosphorus budgets challenge sustainable intensification of grasslands. Nat. Commun. 2016, 7, 10696. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, K.M.; Jefferies, R.L. Vegetation loss alters soil nitrogen dynamics in an Arctic salt marsh. J. Ecol. 2007, 95, 283–293. [Google Scholar] [CrossRef]
- Chen, Q.; Hooper, D.U.; Li, H.; Gong, X.Y.; Peng, F.; Wang, H.; Dittert, K.; Lin, S. Effects of resource addition on recovery of production and plant functional composition in degraded semiarid grasslands. Oecologia 2017, 184, 13–24. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X. Nitrogen deposition weakens plant–microbe interactions in grassland ecosystems. Glob. Change Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef]
- Chen, Q.; Hooper, D.U.; Lin, S. Shifts in Species Composition Constrain Restoration of Overgrazed Grassland Using Nitrogen Fertilization in Inner Mongolian Steppe, China. PLoS ONE 2011, 6, e16909. [Google Scholar] [CrossRef]
- Gough, L.; Gross, K.L.; Cleland, E.E.; Clark, C.M.; Collins, S.L.; Fargione, J.E.; Pennings, S.C.; Suding, K.N. Incorporating clonal growth form clarifies the role of plant height in response to nitrogen addition. Oecologia 2012, 169, 1053–1062. [Google Scholar] [CrossRef]
- Wedin, D.; Tilman, D. Competition Among Grasses Along a Nitrogen Gradient: Initial Conditions and Mechanisms of Competition. Ecol. Monogr. 1993, 63, 199–229. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Sun, S.; Li, S.; Avera, B.N.; Strahm, B.D.; Badgley, B.D.; Löffler, F.E. Soil Bacterial and Fungal Communities Show Distinct Recovery Patterns during Forest Ecosystem Restoration. Appl. Environ. Microbiol. 2017, 83, e00966-17. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Peay, K.G.; Baraloto, C.; Fine, P.V. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Crews, T.E.; Farrington, H.; Vitousek, P.M. Changes in Asymbiotic, Heterotrophic Nitrogen Fixation on Leaf Litter of Metrosideros polymorpha with Long-Term Ecosystem Development in Hawaii. Ecosystems 2000, 3, 386–395. [Google Scholar] [CrossRef]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 2018, 319, 156–166. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Tognetti, P.M.; Prober, S.M.; Báez, S.; Sankaran, M. Negative effects of nitrogen override positive effects of phosphorus on grassland legumes worldwide. Proc. Natl. Acad. Sci. USA 2021, 118, e2023718118. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.; Guohan, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Change Biol. 2010, 16, 889. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the Law of the Minimum. New Phytol. 2014, 205, 1473–1484. [Google Scholar] [CrossRef]
- Gerke, J. Improving Phosphate Acquisition from Soil via Higher Plants While Approaching Peak Phosphorus Worldwide: A Critical Review of Current Concepts and Misconceptions. Plants 2024, 13, 3478. [Google Scholar] [CrossRef] [PubMed]
- Harpole, W.S.; Sullivan, L.L.; Lind, E.M.; Firn, J.; Adler, P.B.; Borer, E.T.; Chase, J.; Fay, P.A.; Hautier, Y.; Hillebrand, H.; et al. Addition of multiple limiting resources reduces grassland diversity. Nature 2016, 537, 93–96. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Lü, X.T.; Stevens, C.J.; Zhang, G.-M.; Wang, H.-Y.; Wang, Z.-W.; Zhang, Z.-J.; Liu, Z.-Y.; Han, X.-G. Mowing mitigates the negative impacts of N addition on plant species diversity. Oecologia 2019, 189, 769–779. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhao, W.; Gao, S.; Wang, R.; Yan, H.; Wang, M. Fertilization Promotes the Recovery of Plant Productivity but Decreases Biodiversity in a Khorchin Degraded Grassland. Nitrogen 2025, 6, 64. https://doi.org/10.3390/nitrogen6030064
Zheng L, Zhao W, Gao S, Wang R, Yan H, Wang M. Fertilization Promotes the Recovery of Plant Productivity but Decreases Biodiversity in a Khorchin Degraded Grassland. Nitrogen. 2025; 6(3):64. https://doi.org/10.3390/nitrogen6030064
Chicago/Turabian StyleZheng, Lina, Wei Zhao, Shaobo Gao, Ruizhen Wang, Haoran Yan, and Mingjiu Wang. 2025. "Fertilization Promotes the Recovery of Plant Productivity but Decreases Biodiversity in a Khorchin Degraded Grassland" Nitrogen 6, no. 3: 64. https://doi.org/10.3390/nitrogen6030064
APA StyleZheng, L., Zhao, W., Gao, S., Wang, R., Yan, H., & Wang, M. (2025). Fertilization Promotes the Recovery of Plant Productivity but Decreases Biodiversity in a Khorchin Degraded Grassland. Nitrogen, 6(3), 64. https://doi.org/10.3390/nitrogen6030064




